|
|
| (15 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| | {{:Team:Johns_Hopkins/Templates/tpl1}} | | {{:Team:Johns_Hopkins/Templates/tpl1}} |
| | <html> | | <html> |
| | + | <br/> |
| | + | <center><table id="Table_01" width="664" height="35" border="0" cellpadding="0" cellspacing="0"> |
| | + | <tr> |
| | + | <td> |
| | + | <a href="https://2011.igem.org/Team:Johns_Hopkins/Notebook/VitExperiments"><img src="https://static.igem.org/mediawiki/2011/0/0a/Menu2vitblack_01.jpg" width="195" height="35" alt=""></a></td> |
| | + | <td> |
| | + | <img src="https://static.igem.org/mediawiki/2011/3/37/Menu2vitblack_02.jpg" width="30" height="35" alt=""></td> |
| | + | <td> |
| | + | <a href="https://2011.igem.org/Team:Johns_Hopkins/Notebook/BCAssay"><img src="https://static.igem.org/mediawiki/2011/6/66/Menu2vit_03.jpg" width="177" height="35" alt=""></a></td> |
| | + | <td> |
| | + | <img src="https://static.igem.org/mediawiki/2011/b/bc/Menu2vitblack_04.jpg" width="29" height="35" alt=""></td> |
| | + | <td> |
| | + | <a href="https://2011.igem.org/Team:Johns_Hopkins/Notebook/DoughMed"><img src="https://static.igem.org/mediawiki/2011/e/e8/Menu2vitblack_05.jpg" width="120" height="35" alt=""></a></td> |
| | + | <td> |
| | + | <img src="https://static.igem.org/mediawiki/2011/d/d0/Menu2vitblack_06.jpg" width="30" height="35" alt=""></td> |
| | + | <td> |
| | + | <a href="https://2011.igem.org/Team:Johns_Hopkins/Notebook/Baking"><img src="https://static.igem.org/mediawiki/2011/7/72/Menu2vitblack_07.jpg" width="83" height="35" alt=""></a></td> |
| | + | </tr> |
| | + | </table></center> |
| | <div id="secondarycontentgreen"> | | <div id="secondarycontentgreen"> |
| | <div id="boxheading"> | | <div id="boxheading"> |
| Line 159: |
Line 178: |
| | |} | | |} |
| | | | |
| | + | Time point 4: 9/16/2011 3pm |
| | + | {| border="1" cellpadding="5" cellspacing="0" style="margin-left:40px;" |
| | + | |- style="background-color:#EBEBE9;" |
| | + | | Strain |
| | + | | OD600 |
| | + | | OD449 |
| | + | |- |
| | + | | WT |
| | + | | 0.176 |
| | + | | 0 |
| | + | |- |
| | + | | 39 |
| | + | | 0.228 |
| | + | | 0.0031 |
| | + | |- |
| | + | | 128 |
| | + | | 0.248 |
| | + | | 0.002 |
| | + | |- |
| | + | | 179 |
| | + | | 0.197 |
| | + | | 0.0042 |
| | + | |- |
| | + | | 201 |
| | + | | 0.257 |
| | + | | 0.038 |
| | + | |- |
| | + | | |
| | + | | 1:100 dil |
| | + | | 990 ul for extraction |
| | + | |} |
| | Time point 5: 9/17/2011 11.30pm | | Time point 5: 9/17/2011 11.30pm |
| | {| border="1" cellpadding="5" cellspacing="0" style="margin-left:40px;" | | {| border="1" cellpadding="5" cellspacing="0" style="margin-left:40px;" |
| Line 189: |
Line 239: |
| | | 1:100 dil | | | 1:100 dil |
| | | 990 ul for extraction | | | 990 ul for extraction |
| | + | |} |
| | + | |
| | + | Time point 6: 9/17/2011 ~2.30pm |
| | + | {| border="1" cellpadding="5" cellspacing="0" style="margin-left:40px;" |
| | + | |- style="background-color:#EBEBE9;" |
| | + | | Strain |
| | + | | OD600 |
| | + | | OD449 |
| | + | |- |
| | + | | WT |
| | + | | 0.150 |
| | + | | 0.002 |
| | + | |- |
| | + | | 39 |
| | + | | 0.024 |
| | + | | 0.028 |
| | + | |- |
| | + | | 128 |
| | + | | 0.250 |
| | + | | 0.006 |
| | + | |- |
| | + | | 179 |
| | + | | 0.269 |
| | + | | 0.028 |
| | + | |- |
| | + | | 201 |
| | + | | 0.292 |
| | + | | 0.055 |
| | + | |- |
| | + | | |
| | + | | 1:100 dil |
| | + | | 740 ul for extraction |
| | + | |} |
| | + | |
| | + | Time point 7: 9/18/2011 ~2pm |
| | + | {| border="1" cellpadding="5" cellspacing="0" style="margin-left:40px;" |
| | + | |- style="background-color:#EBEBE9;" |
| | + | | Strain |
| | + | | OD600 |
| | + | | OD449 |
| | + | |- |
| | + | | WT |
| | + | | 0.104 |
| | + | | 0.001 |
| | + | |- |
| | + | | 39 |
| | + | | 0.210 |
| | + | | 0.022 |
| | + | |- |
| | + | | 128 |
| | + | | 0.233 |
| | + | | 0.01 |
| | + | |- |
| | + | | 179 |
| | + | | 0.213 |
| | + | | 0.02 |
| | + | |- |
| | + | | 201 |
| | + | | 0.209 |
| | + | | 0.041 |
| | + | |- |
| | + | | |
| | + | | 1:100 dil |
| | + | | 740 ul for extraction |
| | |} | | |} |
| | | | |
| | =====9/19/2011===== | | =====9/19/2011===== |
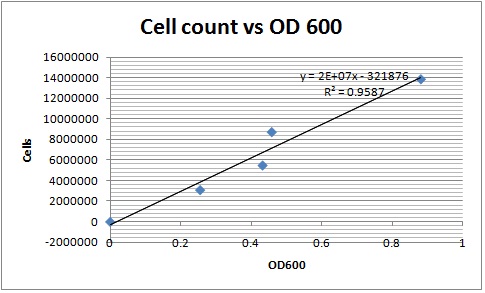
| - | Created a calibration curve was used to estimate the number of cells by counting the number of cells counted on a hemocytometer was plotting it against their absorbance. Cell counts at different time points are then compared to it. Beta carotene in hexane was dried in a vacuum desiccator and stored for future use. | + | [[File:Cell calibration.jpg|thumb]] |
| | + | Created a calibration curve that was used to estimate the number of cells. Counted the number of cells on a hemocytometer and plotted it against the absorbance. Cell counts at different time points were then compared to it. Beta carotene in hexane was dried in a vacuum desiccator and stored for future use. |
| | | | |
| | =====9/23/2011===== | | =====9/23/2011===== |
| - | Ran HPLC analysis on carotenoid extracts | + | Ran HPLC analysis on carotenoid extracts overnight. |
| | + | |
| | + | =====9/24/2011===== |
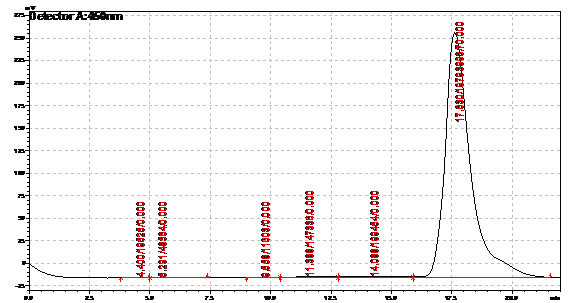
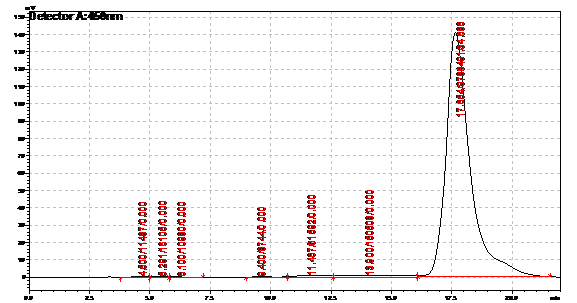
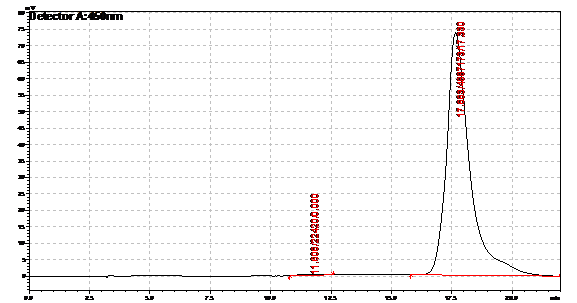
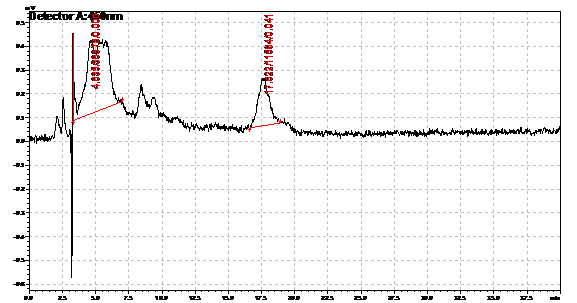
| | + | Obtained results from HPLC. Plotted a standard curve using the beta carotene standards, and compared samples to it. |
| | + | |
| | + | <gallery> |
| | + | File:Hplc calibration.png|Calibration curve |
| | + | File:Standard 2.png|Beta carotene standard, 70ug/ml |
| | + | File:Standard 3.png|Beta carotene standard, 35ug/ml |
| | + | File:Standard 4.png|Beta carotene standard, 17.5ug/ml |
| | + | File:Standard 5.png|Beta carotene standard, 8.75ug/ml |
| | + | </gallery> |
| | + | |
| | + | <gallery> |
| | + | File:2-1.png|strain 39, time point 1 |
| | + | File:2-2.png|strain 39, time point 2 |
| | + | File:2-3.png|strain 39, time point 3 |
| | + | File:2-5.png|strain 39, time point 5 |
| | + | File:2-6.png|strain 39, time point 6 |
| | + | </gallery> |
| | + | |
| | + | <gallery> |
| | + | File:4-1.png|strain 179, time point 1 |
| | + | File:4-2.png|strain 179, time point 2 |
| | + | File:4-3.png|strain 179, time point 3 |
| | + | File:4-5.png|strain 179, time point 5 |
| | + | </gallery> |
| | + | |
| | + | <gallery> |
| | + | File:5-1.png|strain 201, time point 1 |
| | + | File:5-2.png|strain 201, time point 2 |
| | + | File:5-3.png|strain 201, time point 3 |
| | + | File:5-4.png|strain 201, time point 4 |
| | + | File:5-5.png|strain 201, time point 5 |
| | + | File:5-6.png|strain 201, time point 6 |
| | + | </gallery> |
| | + | |
| | + | =====9/25/2011===== |
| | + | First take on extracting beta carotene from bread. |
| | + | |
| | + | =====9/26/2011===== |
| | + | Repeated extraction for both bread baked with wild type and beta carotene producing yeasts. |
| | + | Around 10g of bread was homogenized with water, 20ml of hexane, 14ml of acetone, and 12ml of ethanol added to solubilize. 8ml of KOH in methanol added to saponify. Another 30ml of hexane and 7ml of toluene added after an hour. 10ml aliquot taken from each sample for HPLC analysis on 9/30. |
| | + | |
| | + | |
| | + | |
| | + | =====10/17/2011===== |
| | + | Ashan: Inoculated tubes with Omar's mCherry transformant yeast colonies and a 4 tubes with a control group of untransformed yeast colonies of the same strains (201, 039, 179, 128). Left overnight in 30 degree shaker. |
| | + | |
| | <html> | | <html> |
| | </div> | | </div> |
9/9/2011 - 9/12/2011
Extracted beta carotene for different time points for analysis. Found out that carotenoids concentration was too low. Troubleshooted and optimized the procedure for extraction. Grew up a new stock of wild type and beta carotene producing yeasts (Strain 39, 128, 179, 201) in liquid YPD.
9/12/2011 7pm Inoculation
| Strain
| OD600
| Hexane OD449
| Stock added (ul)
| YPD volume (ml)
|
| WT
| 0.044
| 0
| 70
| 20
|
| 39
| 0.156
| 0
| 285
| 20
|
| 128
| 0.205
| 0
| 285
| 20
|
| 179
| 0.212
| 0
| 285
| 20
|
| 201
| 0.192
| 0
| 285
| 20
|
9/13/2011 - 9/18/2011
Took aliquots at different time points to determine the cell count and amount of beta carotene produced. The cell count was determined by measuring the optical density of the samples at 600nm.
After addition of pyrogallol in methanol, cells were saponified with potassium hydroxide. The samples were incubated at 75 degrees for an hour. Beta carotene was then extracted by adding hexane to the samples and shaking them vigorously. Aliquots were then taken for spectroscopy and for drying.
Concentration of beta carotene in hexane was determined from absorbance at 449nm on a UV spectrophotometer.
Time point 1: 9/13/2011 2pm
| Strain
| OD600
| OD449
|
| WT
| 0.895
| 0
|
| 39
| 0.228
| 0
|
| 128
| 0.226
| 0
|
| 179
| 0.105
| 0
|
| 201
| 0.201
| 0.061
|
|
| 1:4 dil
| 625 ul for extraction
|
Time point 2: 9/14/2011 3pm
| Strain
| OD600
| OD449
|
| WT
| 1.137
| 0
|
| 39
| 1.158
| 0.003
|
| 128
| 1.256
| 0.493
|
| 179
| 0.493
| 0.005
|
| 201
| 1.248
| 0.008
|
|
| 1:10 dil
| 900 ul for extraction
|
Time point 3: 9/15/2011 12.30pm
| Strain
| OD600
| OD449
|
| WT
| 0.364
| 0
|
| 39
| 0.094
| 0.011
|
| 128
| 0.106
| 0
|
| 179
| 0.422
| 0.02
|
| 201
| 0.107
| 0.01
|
|
| 1:50 dil
| 980 ul for extraction
|
Time point 4: 9/16/2011 3pm
| Strain
| OD600
| OD449
|
| WT
| 0.176
| 0
|
| 39
| 0.228
| 0.0031
|
| 128
| 0.248
| 0.002
|
| 179
| 0.197
| 0.0042
|
| 201
| 0.257
| 0.038
|
|
| 1:100 dil
| 990 ul for extraction
|
Time point 5: 9/17/2011 11.30pm
| Strain
| OD600
| OD449
|
| WT
| 0.202
| 0.004
|
| 39
| 0.318
| 0.0032
|
| 128
| 0.253
| 0.017
|
| 179
| 0.227
| 0.048
|
| 201
| 0.277
| 0.102
|
|
| 1:100 dil
| 990 ul for extraction
|
Time point 6: 9/17/2011 ~2.30pm
| Strain
| OD600
| OD449
|
| WT
| 0.150
| 0.002
|
| 39
| 0.024
| 0.028
|
| 128
| 0.250
| 0.006
|
| 179
| 0.269
| 0.028
|
| 201
| 0.292
| 0.055
|
|
| 1:100 dil
| 740 ul for extraction
|
Time point 7: 9/18/2011 ~2pm
| Strain
| OD600
| OD449
|
| WT
| 0.104
| 0.001
|
| 39
| 0.210
| 0.022
|
| 128
| 0.233
| 0.01
|
| 179
| 0.213
| 0.02
|
| 201
| 0.209
| 0.041
|
|
| 1:100 dil
| 740 ul for extraction
|
9/19/2011
Created a calibration curve that was used to estimate the number of cells. Counted the number of cells on a hemocytometer and plotted it against the absorbance. Cell counts at different time points were then compared to it. Beta carotene in hexane was dried in a vacuum desiccator and stored for future use.
9/23/2011
Ran HPLC analysis on carotenoid extracts overnight.
9/24/2011
Obtained results from HPLC. Plotted a standard curve using the beta carotene standards, and compared samples to it.
|
Beta carotene standard, 70ug/ml
|
Beta carotene standard, 35ug/ml
|
Beta carotene standard, 17.5ug/ml
|
Beta carotene standard, 8.75ug/ml
|
9/25/2011
First take on extracting beta carotene from bread.
9/26/2011
Repeated extraction for both bread baked with wild type and beta carotene producing yeasts.
Around 10g of bread was homogenized with water, 20ml of hexane, 14ml of acetone, and 12ml of ethanol added to solubilize. 8ml of KOH in methanol added to saponify. Another 30ml of hexane and 7ml of toluene added after an hour. 10ml aliquot taken from each sample for HPLC analysis on 9/30.
10/17/2011
Ashan: Inoculated tubes with Omar's mCherry transformant yeast colonies and a 4 tubes with a control group of untransformed yeast colonies of the same strains (201, 039, 179, 128). Left overnight in 30 degree shaker.
 "
"