Team:Freiburg/Results
From 2011.igem.org
(Difference between revisions)
SophieCramer (Talk | contribs) (→Plastic binding domain) |
Juliimapril (Talk | contribs) (→Experimental setup) |
||
| (42 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
=results= | =results= | ||
==<span style="color:grey;">Precipitator</span>== | ==<span style="color:grey;">Precipitator</span>== | ||
| - | + | ||
| - | + | ||
[http://partsregistry.org/Part:BBa_K608406 BBa_K608406] | [http://partsregistry.org/Part:BBa_K608406 BBa_K608406] | ||
| Line 77: | Line 76: | ||
| - | '''Precipitator fused with GST tag''' | + | |
| + | ===[https://2011.igem.org/Team:Freiburg/Modelling#A_mathematical_model_to_determine_the_experimental_design Mathematical modeling]=== | ||
| + | |||
| + | To determine the Affinity k_D, experiments to find out the binding affinity of the plastic binding domain are necessary. To get a direct access to these values, we cloned the plastic binding domain in front of a GFP. Then, dilution and washing assays could be performed on polystyrene microtiter plates, red out by a fluorescence plate reader. | ||
| + | The desired parameters could be calculated by measuring dilution rows of GFP proteins and measuring the fluorescence signals at the different concentrations. C_total could be determined by a dilution row with subsequent washing steps, to find out at what [P] concentration there is a saturation. See description of the plastic binding subproject for more detailed explanation on the experimental setup. | ||
| + | |||
| + | A qualitative experiment to prove that Nickel is binding the Precipitator is sufficient, since k_2 >> 1 and does not play a significant role in our setup. This experiment could have been done using a nanofilter that blocks protein but let through ions. The Nickel concentration of the flow through can then be measured. | ||
| + | |||
| + | Alternatively purification of the Precipitator by fusing it with a GST-tag could be done, to subsequently measure the absorbance of the protein, before and after adding Nickel to the solution. After Jordan 1974 a detectable change in the absorbance should be detectable after the complex is formed. A similar effect – a colorshift from white to blue - is visible when one prepares a Ni-NTA column. For this purpose we cloned the GST domain in front of the Precipitator. However towards the end of the project there was no more time to perfom these experiments. | ||
| + | |||
| + | ==References== | ||
| + | |||
| + | |||
| + | Tiandi Wei et.al.; "LRRML: a conformational database and an XML description of | ||
| + | leucine-rich repeats (LRRs)"; BMC Structural Biology 2008 doi: 10.1186/1472-6807-8-47 | ||
| + | |||
| + | |||
| + | Schmidt, Marc et.al.; "Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel"; | ||
| + | Nature 2010 | ||
| + | |||
| + | |||
| + | Letter, J.E. et.al.; "Complexing of Nickel( 11) by Cysteine, Tyrosine, | ||
| + | and Related Ligands and Evidence for Zwitterion Reactivity"; | ||
| + | Contribution from the Department of Chemistry, University of Alberta, | ||
| + | Edmonton, Alberta T6G 2G2, Canada. | ||
| + | |||
| + | |||
| + | Kajava, A.V.;"Structural Diversity of Leucine-rich Repeat Proteins" | ||
| + | J. Mol. Biol. (1998) 277, 519±527 | ||
| + | |||
| + | |||
| + | Kim, Ho Min et. al.; "Crystal Structure of the TLR4-MD-2 Complex with Bound Endotoxin Antagonist Eritoran" | ||
| + | DOI 10.1016/j.cell.2007.08.002 | ||
| + | |||
| + | ==='''Precipitator fused with GST tag'''=== | ||
The Precipitator was fused with the C-terminus of our GST tag in order to extract and further test the construct for its Nickel binding affinity. We successfully cloned it together using the Gibson Assembly and then subsequently pasted it into the iGEM vector. The submitted sequence was partially confirmed by sequencing. | The Precipitator was fused with the C-terminus of our GST tag in order to extract and further test the construct for its Nickel binding affinity. We successfully cloned it together using the Gibson Assembly and then subsequently pasted it into the iGEM vector. The submitted sequence was partially confirmed by sequencing. | ||
| Line 204: | Line 237: | ||
Sandra Harper, David W. Speicher (2008); „Expression and Purification of GST Fusion Proteins“; John Wiley and Sons, Inc.; DOI: 10.1002/0471140864.ps0606s52 | Sandra Harper, David W. Speicher (2008); „Expression and Purification of GST Fusion Proteins“; John Wiley and Sons, Inc.; DOI: 10.1002/0471140864.ps0606s52 | ||
| + | |||
===<span style="color:grey;">Plastic binding domain</span>=== | ===<span style="color:grey;">Plastic binding domain</span>=== | ||
| + | [http://partsregistry.org/Part:BBa_K608404 BBa_K608404] | ||
| + | IPTG-inducible Promoter with plastic binding domain-tagged GFP | ||
| + | ====Experimental setup==== | ||
| + | According to [https://2011.igem.org/Team:Freiburg/Description#Plastic_binding_domain Adey et al.] the plastic binding domain (pbd) binds to the polystyrene surface of micro titer plates (96 well plates). To investigate the binding properties of the plastic binding tag we started several spectroscopic assays using a plate reader (FLUOstar Omega) and polystyrene plates (Greiner bio one) To detect the fluorescence of the GFP tagged to the plastic binding domain we used black plates and a well scanning program measuring 10x10 spots in each well of the micro titer plate. We performed several washing steps to find out how much of the proteins can be found in the eluate and how much remains bound on the plate’s surface. To compare the pbd-tagged GFP to a normal GFP without special plastic binding ability we also measured GFP obtained via expression with our diverse PR (Promoter-Ribosome-binding-site constructs). To affirm the results obtained by fluorescence spectroscopy used the Bradford assay and screened for different protein concentrations in transparent polystyrene plates. | ||
| + | The calibration lines necessary for calculation of protein concentration can be found [[Media:Bradford+Fluorescence calibration.pdf|here]]. | ||
| + | |||
| + | ====Results==== | ||
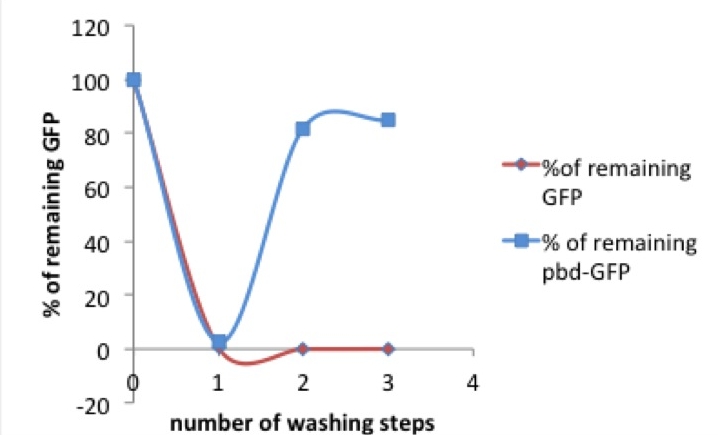
[[Image:picture 1 pbd.jpg|250px|right|thumb|Picture 1: % of tagged (red) or untagged (blue) GFP remaining in the well after washing compared to previous washing step / original concentration]] After the first measurement of the basic fluorescence intensity we transferred the samples onto another well, refilled the exhausted well with PBS and measured both eluate and remaining protein. In a second washing step the liquid was taken out of the first well again and given to another well. This washing was performed a third time, resulting in three eluates and a triply washed well with more or less protein remaining on the walls. | [[Image:picture 1 pbd.jpg|250px|right|thumb|Picture 1: % of tagged (red) or untagged (blue) GFP remaining in the well after washing compared to previous washing step / original concentration]] After the first measurement of the basic fluorescence intensity we transferred the samples onto another well, refilled the exhausted well with PBS and measured both eluate and remaining protein. In a second washing step the liquid was taken out of the first well again and given to another well. This washing was performed a third time, resulting in three eluates and a triply washed well with more or less protein remaining on the walls. | ||
<br/> | <br/> | ||
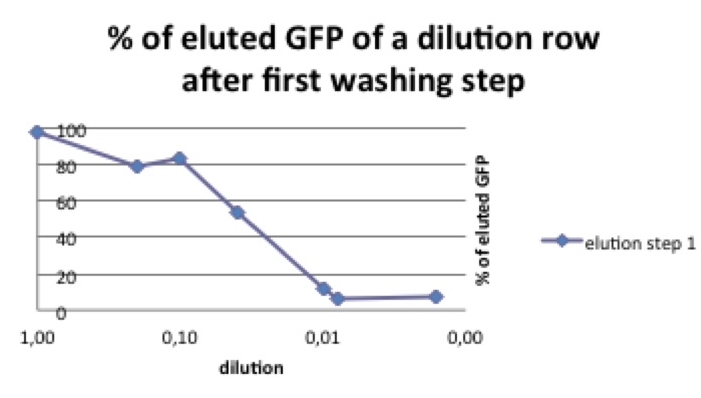
| - | As shown in picture 1, both pbd-tagged and untagged GFP fluorescence decreases after the first washing step. Only 0- 4% of the original concentrations of the untagged GFP and 2-7% of the pbd-tagged GFP remained in the well.[[Image:picture 2 pbd.jpg|250px|right|thumb|Picture 2: % of pbd-tagged GFP that can be eluted in the first washing step. The curve decreases with the amount of the start concentration. If the GFP-pbd solution is not oversaturated a lower amount of this protein can be washed away.]] After a second washing step there were differences observable between the tagged and the untagged GFP. While the percentage of the remaining “normal” GFP was | + | As shown in picture 1, both pbd-tagged and untagged GFP fluorescence decreases after the first washing step. Only 0- 4% of the original concentrations of the untagged GFP and 2-7% of the pbd-tagged GFP remained in the well.[[Image:picture 2 pbd.jpg|250px|right|thumb|Picture 2: % of pbd-tagged GFP that can be eluted in the first washing step. The curve decreases with the amount of the start concentration. If the GFP-pbd solution is not oversaturated a lower amount of this protein can be washed away.]] After a second washing step there were differences observable between the tagged and the untagged GFP. While the percentage of the remaining “normal” GFP was scattered around zero, an average of 60% of the pbd-tagged GFP remained in the well. In a third washing step, this observation was confirmed. While, like before, the main part of the pbd-GFP remained in its original well, nearly all of the untagged GFP was washed off. |
<br/> | <br/> | ||
| - | + | Taking a look at the pbd-bound GFP, it is striking that after the solution has already been diluted by one washing step, the percentage of protein that can be washed away diminishes. It comes to mind that the massive loss of pbd-tagged GFP after the first washing step might be due to an oversaturation of pbd-GFP in the solution. In this case there would be too much pbd-GFP for the available plastic surface, so that most of the protein could be eluated. | |
| + | <br/> | ||
| + | As shown in picture 2 the percentage of eluted GFP diminishes when the used start concentration is lower. [[Image: pbd_better_than_GFP.jpg|250px|right| thumb | Picture 3: if the start concentration is not oversaturated pbd-coupled is much more resistant to washing steps than GFP alone.]] | ||
<br/> | <br/> | ||
| - | |||
To investigate this phenomenon we compared different start concentrations of pbd-GFP concerning the amount of pbd-GFP that could be washed away. | To investigate this phenomenon we compared different start concentrations of pbd-GFP concerning the amount of pbd-GFP that could be washed away. | ||
| - | polystyrene micro titer plates provide only a limited surface for the pbd to bind, the solution shouldn’t be oversaturated with plastic binding protein. | + | polystyrene micro titer plates provide only a limited surface for the pbd to bind, the solution shouldn’t be oversaturated with plastic binding protein. In our case the diluted protein could only reach a surface of 91.5 mm2 per well. With a remaining protein amount of ~220 pg/well after three washing steps we estimate that 2,4 pg pbd-tagged protein can be bound per mm2. |
<br/> | <br/> | ||
| - | In a range of 1-30ng/µL start concentration there remains about seven times more pbd-GFP after washing than "normal" GFP (see picture 3). | + | In a range of 1-30ng/µL start concentration there remains about seven times more pbd-GFP after washing than "normal" GFP (see picture 3). |
| - | |||
| - | |||
<br/> | <br/> | ||
====Discussion==== | ====Discussion==== | ||
| - | + | In the experiments described above we could show that in a certain range of start concentration the plastic binding domain (pbd)-coupled GFP was more resistant to elution steps than GFP alone. This result indicates that the pbd-tagged GFP bound stronger to the plastic surface of the microtiter plate than “normal” GFP. The fact that in case of a rather high start concentration the majority of the pbd-GFP was washed away can be explained by the limitation of available binding surface. It seems like the pbd-GFP binds seven times better to the used polystyrene material than GFP alone. The amount of pbd-GFP that can bind to 1 mm2 is about 2,4 pg. To prove this supposition further measurement and more washing steps should be performed. | |
| Line 237: | Line 277: | ||
We could successfully amplify the gene sequences for ''CcaS'' and ''CcaR'' and the promotor region ''PcpcG'' via <br/>PCR from the whole ''Synechocystis sp.'' PCC6803 genome.<br/> | We could successfully amplify the gene sequences for ''CcaS'' and ''CcaR'' and the promotor region ''PcpcG'' via <br/>PCR from the whole ''Synechocystis sp.'' PCC6803 genome.<br/> | ||
Later two parts ([http://partsregistry.org/Part:BBa_K608101 BBa_K608101] , [http://partsregistry.org/Part:BBa_K608102 BBa_K608102]), were inserted into the iGEM-Vector pSB1C3 and send to the registry.<br/> | Later two parts ([http://partsregistry.org/Part:BBa_K608101 BBa_K608101] , [http://partsregistry.org/Part:BBa_K608102 BBa_K608102]), were inserted into the iGEM-Vector pSB1C3 and send to the registry.<br/> | ||
| - | Twice we tried to clone the promotor region ''PcpcG'' into a vector, but had no insert at all. | + | Twice we tried to clone the promotor region ''PcpcG'' into a vector, but had no insert at all.<br/> |
| - | |||
After a summer full of cloning we ran out of time to assemble <br/> | After a summer full of cloning we ran out of time to assemble <br/> | ||
the green light receptor cassette like we planned to.<br/> | the green light receptor cassette like we planned to.<br/> | ||
| + | For this reason we could not test the green light receptor and can not provide more information.<br/> | ||
==<span style="color:red;">red light receptor</span>== | ==<span style="color:red;">red light receptor</span>== | ||
| Line 306: | Line 346: | ||
==<span style="color:grey;">Precipitator</span>== | ==<span style="color:grey;">Precipitator</span>== | ||
[http://partsregistry.org/Part:BBa_K608404 BBa_K608404] | [http://partsregistry.org/Part:BBa_K608404 BBa_K608404] | ||
| - | IPTG-inducible Promoter with plastic binding domain-tagged GFP | + | IPTG-inducible Promoter with '''plastic binding domain'''-tagged GFP |
[http://partsregistry.org/Part:BBa_K608406 BBa_K608406] | [http://partsregistry.org/Part:BBa_K608406 BBa_K608406] | ||
| Line 356: | Line 396: | ||
[http://partsregistry.org/Part:BBa_K608002 BBa_K608002] | [http://partsregistry.org/Part:BBa_K608002 BBa_K608002] | ||
| - | strong Promoter and strong RBS | + | strong Promoter and strong RBS (PR1) |
[http://partsregistry.org/Part:BBa_K608003 BBa_K608003] | [http://partsregistry.org/Part:BBa_K608003 BBa_K608003] | ||
| - | strong Promoter , medium RBS | + | strong Promoter , medium RBS (PR2) |
[http://partsregistry.org/Part:BBa_K608004 BBa_K608004] | [http://partsregistry.org/Part:BBa_K608004 BBa_K608004] | ||
| - | strong Promoter , weak RBS | + | strong Promoter , weak RBS (PR3) |
[http://partsregistry.org/Part:BBa_K608005 BBa_K608005] | [http://partsregistry.org/Part:BBa_K608005 BBa_K608005] | ||
| - | medium Promoter , strong RBS | + | medium Promoter , strong RBS (PR4) |
[http://partsregistry.org/Part:BBa_K608006 BBa_K608006] | [http://partsregistry.org/Part:BBa_K608006 BBa_K608006] | ||
| - | medium Promoter , medium RBS | + | medium Promoter , medium RBS (PR5) |
[http://partsregistry.org/Part:BBa_K608007 BBa_K608007] | [http://partsregistry.org/Part:BBa_K608007 BBa_K608007] | ||
| - | medium Promoter , weak RBS | + | medium Promoter , weak RBS (PR6) |
| + | |||
| + | |||
| + | |||
| + | |||
| + | '''Measurement of fluorescence intensity of PR-GFP and PR-RFP''' | ||
---- | ---- | ||
| Line 378: | Line 423: | ||
To quantify the strength of our PR-constructs we cloned GFP and RFP behind it<br/> | To quantify the strength of our PR-constructs we cloned GFP and RFP behind it<br/> | ||
and quantified the protein output.<br/> | and quantified the protein output.<br/> | ||
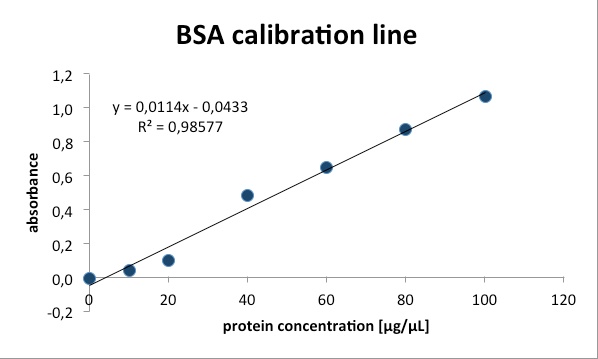
| - | + | [[File:Freiburg2011_BSA.jpg|400px|right|thumb|BSA calibration line]] | |
The fluorescence was measured with a plate reader:<br/> | The fluorescence was measured with a plate reader:<br/> | ||
The fluorescence intensity and protein concentration were measured with the FLUOstar Omega, <br/> | The fluorescence intensity and protein concentration were measured with the FLUOstar Omega, <br/> | ||
| Line 385: | Line 430: | ||
We measured the protein concentration with the bradford-assay. This is a method to determine the total protein concentration. To analyze the protein concentration of the samples, Coomassie Brillant Blue was pippeted to each sample. With the binding of the dye to the proteins the color changes from dark red to blue. The more protein in the solution the more Coomassie dye can bind to proteins and the more the color changes into blue. The absorption of bound Coomassie dye is 595nm. The absorbance is proportional with the amount of bound dye. With a series of Bovine Serum Albumin (BSA) measurements the exact protein concentration of the samples can be determined. BSA acts like a “marker” because the concentration of BSA is known and with a linear calibration line the exact protein concentration can be detected. | We measured the protein concentration with the bradford-assay. This is a method to determine the total protein concentration. To analyze the protein concentration of the samples, Coomassie Brillant Blue was pippeted to each sample. With the binding of the dye to the proteins the color changes from dark red to blue. The more protein in the solution the more Coomassie dye can bind to proteins and the more the color changes into blue. The absorption of bound Coomassie dye is 595nm. The absorbance is proportional with the amount of bound dye. With a series of Bovine Serum Albumin (BSA) measurements the exact protein concentration of the samples can be determined. BSA acts like a “marker” because the concentration of BSA is known and with a linear calibration line the exact protein concentration can be detected. | ||
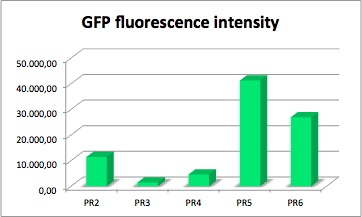
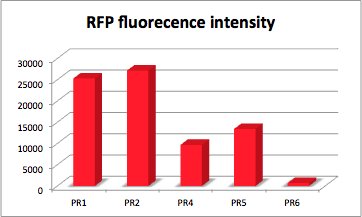
| + | The values of the fluorescence intensity of RFP and GFP deviate from the expected values. We´ve expected PR1 to have the strongest GFP/RFP signal and PR6 with the lowest GFP/RFP signal. The data shows the differences. We could not repeat this measurement another time because of lack of time. We could not measure PR1-GFP because the sequencing was negative and there was no time for repeating. The measurement of PR3-RFP was an error and was excluded. | ||
| - | GFP | + | '''PR-GFP''' |
| + | ---- | ||
| - | [[File: | + | [[File:Freiburg2011_GFP1.jpg|400px|thumb|right|GFP fluorescence intensity dependent on the strenght of promoter and RBS.]] |
| + | GFP served as a reporter of expression. We wanted to know how strong the promoter and RBS activity is. With this reporter gene it was possible to analyze the expression via plate reader. GFP is excited at a wavelength of 509nm and has an emission of 520nm. The plate reader illuminates the samples with a high energy xenon flash lamp. Optical filters or monochromator create the exact wavelength. The more GFP in the sample the higher is the GFP fluorescence intensity. The intensity is collected with the second optical system and is detected with a side window photomultiplier tube. | ||
| + | '''parts:'''<br/> | ||
[http://partsregistry.org/Part:BBa_K608008 BBa_K608008] | [http://partsregistry.org/Part:BBa_K608008 BBa_K608008] | ||
| - | constitutive strong promoter with medium RBS and GFP | + | constitutive strong promoter with medium RBS and GFP (PR2-GFP) |
[http://partsregistry.org/Part:BBa_K608009 BBa_K608009] | [http://partsregistry.org/Part:BBa_K608009 BBa_K608009] | ||
| - | Strong promoter with weak RBS and GFP | + | Strong promoter with weak RBS and GFP (PR3-GFP) |
[http://partsregistry.org/Part:BBa_K608010 BBa_K608010] | [http://partsregistry.org/Part:BBa_K608010 BBa_K608010] | ||
| - | Medium promoter with strong RBS and GFP | + | Medium promoter with strong RBS and GFP (PR4-GFP) |
[http://partsregistry.org/Part:BBa_K608011 BBa_K608011] | [http://partsregistry.org/Part:BBa_K608011 BBa_K608011] | ||
| - | Medium promoter with medium RBS and GFP | + | Medium promoter with medium RBS and GFP (PR5-GFP) |
[http://partsregistry.org/Part:BBa_K608012 BBa_K608012] | [http://partsregistry.org/Part:BBa_K608012 BBa_K608012] | ||
| - | Medium promoter with weak RBS and GFP | + | Medium promoter with weak RBS and GFP (PR6-GFP) |
| - | |||
| + | |||
| + | |||
| + | '''PR-RFP''' | ||
| + | ---- | ||
| + | [[File:Freiburg2011_RFP.jpg|400px|thumb|right|RFP fluorescence intensity dependent on the strength of promotor and RBS.]] | ||
With RFP (Red Fluorescence Protein) the activity of promoter and RBS can be quantified. | With RFP (Red Fluorescence Protein) the activity of promoter and RBS can be quantified. | ||
RFP served like GFP as a reporter to determine the expression level. RFP is excited at a wavelength of 580nm. The more RFP in the solution the more is the RFP fluorescence intensity.The plate reader illuminates the samples with a high energy xenon flash lamp. Optical filters or monochromator create the exact wavelength. The more RFP in the sample the higher is the RFP fluorescence intensity. The intensity is collected with the second optical system and is detected with a side window photomultiplier tube. | RFP served like GFP as a reporter to determine the expression level. RFP is excited at a wavelength of 580nm. The more RFP in the solution the more is the RFP fluorescence intensity.The plate reader illuminates the samples with a high energy xenon flash lamp. Optical filters or monochromator create the exact wavelength. The more RFP in the sample the higher is the RFP fluorescence intensity. The intensity is collected with the second optical system and is detected with a side window photomultiplier tube. | ||
| + | |||
| + | '''parts:'''<br/> | ||
[http://partsregistry.org/Part:BBa_K608013 BBa_K608013] | [http://partsregistry.org/Part:BBa_K608013 BBa_K608013] | ||
| - | strong Promoter with strong RBS and RFP | + | strong Promoter with strong RBS and RFP (PR1-RFP) |
[http://partsregistry.org/Part:BBa_K608014 BBa_K608014 ] | [http://partsregistry.org/Part:BBa_K608014 BBa_K608014 ] | ||
| - | PR with RFP | + | PR with RFP (PR2-RFP) |
[http://partsregistry.org/Part:BBa_K608015 BBa_K608015] | [http://partsregistry.org/Part:BBa_K608015 BBa_K608015] | ||
| - | PR with RFP | + | PR with RFP (PR3-RFP) |
[http://partsregistry.org/Part:BBa_K608016 BBa_K608016] | [http://partsregistry.org/Part:BBa_K608016 BBa_K608016] | ||
| - | PR with RFP | + | PR with RFP (PR4-RFP) |
[http://partsregistry.org/Part:BBa_K608017 BBa_K608017] | [http://partsregistry.org/Part:BBa_K608017 BBa_K608017] | ||
| - | PR with RFP | + | PR with RFP (PR5-RFP) |
[http://partsregistry.org/Part:BBa_K608018 BBa_K608018] | [http://partsregistry.org/Part:BBa_K608018 BBa_K608018] | ||
| - | PR with RFP | + | PR with RFP (PR6-RFP) |
{{:Team:Freiburg/Templates/footer}} | {{:Team:Freiburg/Templates/footer}} | ||
Latest revision as of 03:54, 22 September 2011
 "
"
 Contact
Contact