Team:Cambridge/Experiments/Periplasmic Export
From 2011.igem.org
(→Assembly of constructs for expression of fusion proteins) |
|||
| (11 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | {{Template:Team:Cambridge/ | + | {{Template:Team:Cambridge/CAM_2011_EXPERIMENT_HEAD}} |
=Periplasmic export= | =Periplasmic export= | ||
| Line 16: | Line 16: | ||
===Assembly of constructs for expression of fusion proteins=== | ===Assembly of constructs for expression of fusion proteins=== | ||
| - | We assembled constructs for export of TorA-Reflectin and TorA-Reflectin-GFP (to give an easy control for reflectin export | + | We assembled [[Team:Cambridge/Experiments/Plasmid_Constructs | constructs]] for export of his-tagged TorA-Reflectin A1 (GA17) and TorA-Reflectin A1-GFP (GA18) in order to give an easy control for reflectin export. |
Sequences of the primers used in the experiment and their predicted melting temperatures are the following: | Sequences of the primers used in the experiment and their predicted melting temperatures are the following: | ||
| Line 38: | Line 38: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>RefA1-His- | + | <td>RefA1-His-pSB-fwd</td> |
| + | <td>AACATCATCACCATCACCACTACTAGAGCCAGGCATCAAATAAAAC | ||
| + | <td>64.41°C</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>RefA1-His-pSB-rev</td> | ||
<td>GCTCTAGTATTAGTGGTGATGGTGATGATGGTACATGTGGTAATCGTAATAATTACGATCG</td> | <td>GCTCTAGTATTAGTGGTGATGGTGATGATGGTACATGTGGTAATCGTAATAATTACGATCG</td> | ||
<td>66.41°C</td> | <td>66.41°C</td> | ||
| Line 44: | Line 49: | ||
<tr> | <tr> | ||
<td>TorA-RefA1-Fwd</td> | <td>TorA-RefA1-Fwd</td> | ||
| - | <td> | + | <td>GCTGTTGACTCCTCGCAGAGCGACGGCAGCACAAGCCGCAATGGGATCCATGAACCGTTACC</td> |
<td>67.89°C</td> | <td>67.89°C</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td> | + | <td>pSB-TorA-Rev</td> |
| - | <td> | + | <td>GAAAGCGACGCCGAGAAGCCTGAAATAAGTCGTTGTTCGCCATCTAGCTACTAGAGAAAGAGGAGAAATACTAG</td> |
| - | <td>60. | + | <td>60.48°C</td> |
</tr> | </tr> | ||
</table> | </table> | ||
</html> | </html> | ||
</center> | </center> | ||
| + | |||
| + | Components of the plasmids amplified by PCR, together with template DNA used, are presented on the diagrams below: | ||
| + | [[File:cam_igem-GA17.png | thumb | center | 700px | GA17 construct]] | ||
| + | [[File:cam_igem-GA18.png | thumb | center | 700px | GA18 construct]] | ||
| + | |||
| + | We joined components of these plasmids using [[Team:Cambridge/Protocols/Gibson_Assembly |Gibson Assembly]], and then checked how correct the assembly was using [[Team:Cambridge/Protocols/Colony_PCR |oolony PCR]] with the [http://partsregistry.org/wiki/index.php?title=Part:BBa_G00100 VF2] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_G00101 VR] standard primers. The bands which we obtained on an agarose gel matched the expected lengths of the constructs, which for GA17 and GA18 are 2900 bp and 3600 bp, respectively. | ||
==GFP imaging== | ==GFP imaging== | ||
| Line 76: | Line 87: | ||
[http://www.ncbi.nlm.nih.gov/pubmed/12711311 ''Barrett et al''] suggests that our level of induction was too high to see any significant export. We aim to try altered growth conditions in the hope of improving export efficiency, but as of the time of writing we have not had a chance to attempt this. | [http://www.ncbi.nlm.nih.gov/pubmed/12711311 ''Barrett et al''] suggests that our level of induction was too high to see any significant export. We aim to try altered growth conditions in the hope of improving export efficiency, but as of the time of writing we have not had a chance to attempt this. | ||
| - | {{Template:Team:Cambridge/ | + | {{Template:Team:Cambridge/CAM_2011_EXPERIMENT_FOOT}} |
Latest revision as of 20:16, 21 September 2011
Contents |
Periplasmic export
As reflectins appear to spontaneously associate with membranes, we hoped to promote correct assembly by directing our recombinant reflectins to the [http://en.wikipedia.org/wiki/Periplasmic_space periplasmic space of E. coli].
Design of constructs
Choice of signal sequences
The signal sequence from TorA [http://www.ncbi.nlm.nih.gov/pubmed/11123687 has been used to export GFP to the periplasm] via the TAT pathway. We focused our export plan on this sequence as the TAT pathway exports proteins in their fully folded state, allowing GFP to remain fluorescent (it cannot form a fluorophore in a reducing environment). This allows us to use our reflectin-GFP fusions to provide an easy reporter of export using a confocal microscope.
The amino acid sequence given by Thomas et al was 4 residues shorter than [http://partsregistry.org/Part:BBa_K233307 part BBa_K233307], allowing us to directly synthesise it more cheaply from two primers. Our revised sequence has been submitted as an improved part.
We also designed reflectin fusions with two other signal sequences, from the PelB and PhoD proteins. We are very grateful to Nick Thompson (Physics of Medicine) and Dr David Summers (Dept of Genetics) for their help and advice with choosing signal sequences, and regret that we did not have time to follow through on their suggestions.
Assembly of constructs for expression of fusion proteins
We assembled constructs for export of his-tagged TorA-Reflectin A1 (GA17) and TorA-Reflectin A1-GFP (GA18) in order to give an easy control for reflectin export.
Sequences of the primers used in the experiment and their predicted melting temperatures are the following:
| Name | Sequence | Tm |
|---|---|---|
| TorA Fwd | ATGGCGAACAACGACTTATTTCAGGCTTCTCGGCGTCGCTTTCTGGCGCAGCTGGGCGGATTAACGGTGGCGGGT | 70.98°C |
| TorA Rev | TGCGGCTTGTGCTGCCGTCGCTCTGCGAGGAGTCAACAGCGACGGGCCCAACATACCCGCCACCGTTAATCCGCC | 70.98°C |
| RefA1-His-pSB-fwd | AACATCATCACCATCACCACTACTAGAGCCAGGCATCAAATAAAAC | 64.41°C |
| RefA1-His-pSB-rev | GCTCTAGTATTAGTGGTGATGGTGATGATGGTACATGTGGTAATCGTAATAATTACGATCG | 66.41°C |
| TorA-RefA1-Fwd | GCTGTTGACTCCTCGCAGAGCGACGGCAGCACAAGCCGCAATGGGATCCATGAACCGTTACC | 67.89°C |
| pSB-TorA-Rev | GAAAGCGACGCCGAGAAGCCTGAAATAAGTCGTTGTTCGCCATCTAGCTACTAGAGAAAGAGGAGAAATACTAG | 60.48°C |
Components of the plasmids amplified by PCR, together with template DNA used, are presented on the diagrams below:
We joined components of these plasmids using Gibson Assembly, and then checked how correct the assembly was using oolony PCR with the [http://partsregistry.org/wiki/index.php?title=Part:BBa_G00100 VF2] and [http://partsregistry.org/wiki/index.php?title=Part:BBa_G00101 VR] standard primers. The bands which we obtained on an agarose gel matched the expected lengths of the constructs, which for GA17 and GA18 are 2900 bp and 3600 bp, respectively.
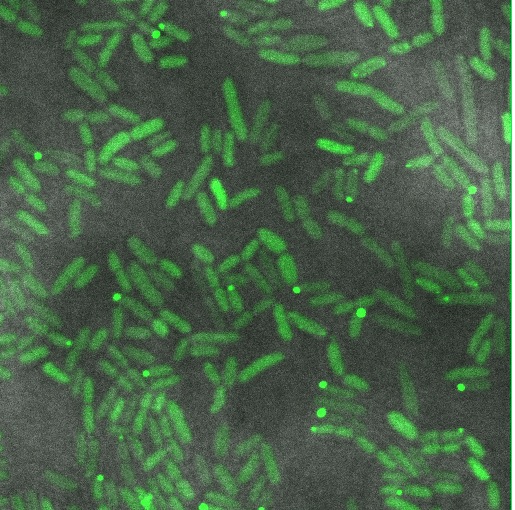
GFP imaging
We hoped to see a halo effect of GFP in the periplasm, demonstrating correct export of our TorA-Reflectin-GFP fusion. Unfortunately, our preliminary observations do not support this.
Instead we see roughly even distribution of GFP throughout the cell, with a few cells forming inclusion bodies.
There are a number of possible reasons why this might be; our torA tag may be dysfunctional, or we might be expressing too strongly and overloading the export machinery. Possible next steps would involve inducing at a lower level, and a torA-GFP fusion as a control.
It was not possible to try these things before the wiki freeze, however if time allows we will refine our protocol to see if reflectins can be exported by this pathway before the jamboree.
Conclusion
Time did not allow us to achieve functional export of reflectin, so we cannot know whether reflectin self assembly will give structural colour in the periplasm.
[http://www.ncbi.nlm.nih.gov/pubmed/12711311 Barrett et al] suggests that our level of induction was too high to see any significant export. We aim to try altered growth conditions in the hope of improving export efficiency, but as of the time of writing we have not had a chance to attempt this.
Back to Experiments
 "
"