Team:Groningen/project AND gate
From 2011.igem.org
JoyceM1013 (Talk | contribs) |
|||
| (19 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{HeaderGroningen2011}} | {{HeaderGroningen2011}} | ||
| - | |||
=AND gate= | =AND gate= | ||
| - | |||
==The engineered riboregulator by Isaacs et al.== | ==The engineered riboregulator by Isaacs et al.== | ||
| - | + | [[File:riboregulator.png|Engineered Riboregulator, modified after Isaacs et al. (Nat Biotechnol., 2004)|right|thumb|350px]] | |
In order to integrate the memory units, we designed an AND gate system which is based on the engineered riboregulator presented by [http://www.ncbi.nlm.nih.gov/pubmed/15208640 Isaacs at al.] in 2004. <br> | In order to integrate the memory units, we designed an AND gate system which is based on the engineered riboregulator presented by [http://www.ncbi.nlm.nih.gov/pubmed/15208640 Isaacs at al.] in 2004. <br> | ||
| - | The design is based on two subparts, a ''cis''-repressed mRNA (crRNA) and a ''trans''-activating RNA. The crRNA is composed of the sequence of the target gene and an upstream short | + | |
| - | The taRNA exhibits partial complementarity to the ''cis''- | + | The design is based on two subparts, a ''cis''-repressed mRNA (crRNA) (1) and a ''trans''-activating RNA (taRNA) (2). The crRNA is composed of the sequence of the target gene and an upstream short nucleotide sequence which is complementary to the ribosome binding site (RBS). Translation inhibition is achieved through sequestration of the RBS by formation of a stem loop structure in the 5'-untranslated region of the mRNA. This prevents binding of the RBS by the 30s ribosomal subunit and subsequent translation. <br> |
| + | The non-coding taRNA exhibits partial complementarity to the ''cis''-repressed sequence. Its expression, which is driven by a second promoter, allows formation of a RNA duplex molecule (3) and is accompanied by a conformational change in the crRNA structure. The stem-loop structure is unfolded, subsequently abolishing RBS obstruction and allowing translation of the target gene. The RBS present in the taRNA sequence is sequestrated by a stem structure. Twenty six nucleotides are involved in the intermolecular interaction, providing 10^15 unique taRNA-crRNA pairs. | ||
| + | |||
<br> | <br> | ||
| - | The system | + | The system possess several advantages over regular RNA interference to control gene expression: First and most importantly, it can repress as well as activate translation. This characteristic is crucial for the functionality of our system, as it will allow reset of our circuit to the initial state. Second, both the modular structure and the functionality with a variety of genes predestine the artificial riboregulator of Isaacs at al. for the use in synthetic biology and, more specifically in our genetic circuit. |
<br> | <br> | ||
==The riboregulator in Count coli== | ==The riboregulator in Count coli== | ||
| - | + | ||
| - | We built two BioBricks which represent our artificial riboregulator and will link the two memory units as an AND gate. <br> | + | [[File:and-gate-scheme.png|The integrated AND gate in our circuit|center|thumb|700px]] |
| - | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K607005 | + | We designed and built two BioBricks which represent our artificial riboregulator and will link the two memory units as an AND gate. <br> |
| - | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K607000 BBa_K607000] is the complementary taRNA with an upstream hybB promoter. | + | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K607005 BBa_K607005] is a combination of a ''cis''-repressive sequence with the coding region of the lasR protein under transcriptional control of the Prm promoter. |
| - | [[ | + | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K607000 BBa_K607000] is the complementary taRNA with an upstream hybB promoter. <br> |
| - | + | We expect the system to work as following: | |
| + | [[File:and-gate.png|Theoretic model of our AND gate|center|thumb|700px]] | ||
| + | The first input will lead to production of taRNA through activation of the PhybB promoter. Simultaneously, via activation of the same promoter, transcription of the cI gene will start. Subsequently, produced cI activates the Prm promoter, which in turn will initiate transcription of crRNA-lasR. As degradation of RNA occurs quite fast, we expect that the time frame of transcription and translation of cI with downstream crRNA-lasR transcription exceeds the lifetime of the taRNA molecules. The first memory unit, once activated by the first input signal, will lead to continuous production of crRNA-lasR, whilst taRNA is absent. After a second input is given, taRNA concentrations will increase again. As now crRNA-lasR is present, formation of a RNA duplex molecule will take place, leading to translation of lasR. This will allow activation of the second memory unit only after the second input signal. | ||
| + | |||
{{FooterGroningen2011}} | {{FooterGroningen2011}} | ||
Latest revision as of 22:05, 21 September 2011
AND gate
The engineered riboregulator by Isaacs et al.
In order to integrate the memory units, we designed an AND gate system which is based on the engineered riboregulator presented by [http://www.ncbi.nlm.nih.gov/pubmed/15208640 Isaacs at al.] in 2004.
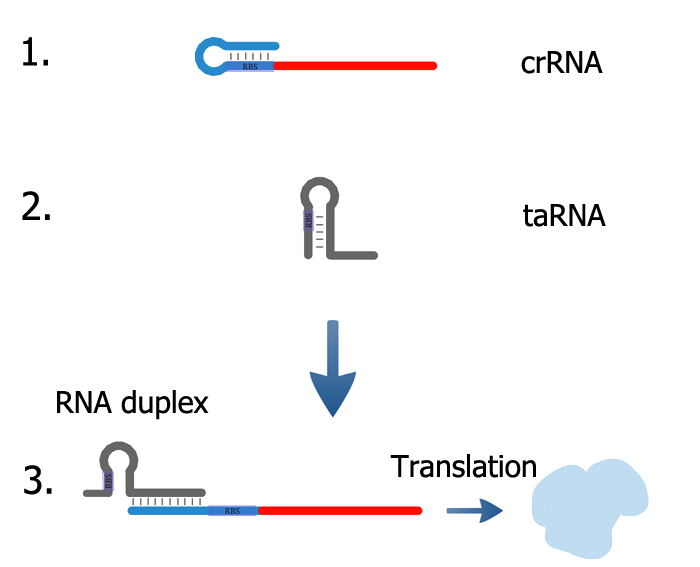
The design is based on two subparts, a cis-repressed mRNA (crRNA) (1) and a trans-activating RNA (taRNA) (2). The crRNA is composed of the sequence of the target gene and an upstream short nucleotide sequence which is complementary to the ribosome binding site (RBS). Translation inhibition is achieved through sequestration of the RBS by formation of a stem loop structure in the 5'-untranslated region of the mRNA. This prevents binding of the RBS by the 30s ribosomal subunit and subsequent translation.
The non-coding taRNA exhibits partial complementarity to the cis-repressed sequence. Its expression, which is driven by a second promoter, allows formation of a RNA duplex molecule (3) and is accompanied by a conformational change in the crRNA structure. The stem-loop structure is unfolded, subsequently abolishing RBS obstruction and allowing translation of the target gene. The RBS present in the taRNA sequence is sequestrated by a stem structure. Twenty six nucleotides are involved in the intermolecular interaction, providing 10^15 unique taRNA-crRNA pairs.
The system possess several advantages over regular RNA interference to control gene expression: First and most importantly, it can repress as well as activate translation. This characteristic is crucial for the functionality of our system, as it will allow reset of our circuit to the initial state. Second, both the modular structure and the functionality with a variety of genes predestine the artificial riboregulator of Isaacs at al. for the use in synthetic biology and, more specifically in our genetic circuit.
The riboregulator in Count coli
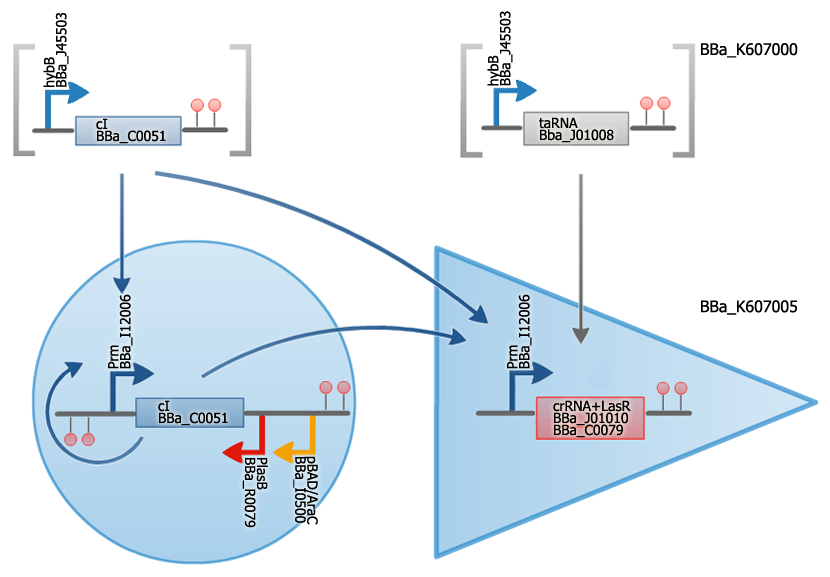
We designed and built two BioBricks which represent our artificial riboregulator and will link the two memory units as an AND gate.
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K607005 BBa_K607005] is a combination of a cis-repressive sequence with the coding region of the lasR protein under transcriptional control of the Prm promoter.
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K607000 BBa_K607000] is the complementary taRNA with an upstream hybB promoter.
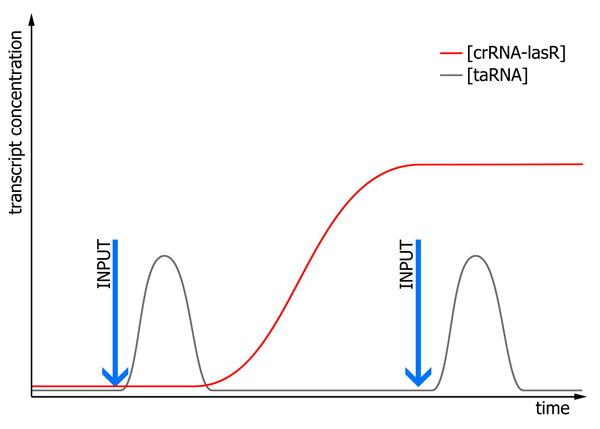
We expect the system to work as following:
The first input will lead to production of taRNA through activation of the PhybB promoter. Simultaneously, via activation of the same promoter, transcription of the cI gene will start. Subsequently, produced cI activates the Prm promoter, which in turn will initiate transcription of crRNA-lasR. As degradation of RNA occurs quite fast, we expect that the time frame of transcription and translation of cI with downstream crRNA-lasR transcription exceeds the lifetime of the taRNA molecules. The first memory unit, once activated by the first input signal, will lead to continuous production of crRNA-lasR, whilst taRNA is absent. After a second input is given, taRNA concentrations will increase again. As now crRNA-lasR is present, formation of a RNA duplex molecule will take place, leading to translation of lasR. This will allow activation of the second memory unit only after the second input signal.
 "
"