Team:Glasgow/Ranaspumin2
From 2011.igem.org
| (2 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Team:Glasgow/Header}} | {{Team:Glasgow/Header}} | ||
<html> | <html> | ||
| - | |||
<body> | <body> | ||
| + | <h1> Ranaspumin-2</h1> | ||
| + | <h6><a href="https://2011.igem.org/Team:Glasgow/Parts">Back to Parts</a></h6> | ||
<p> | <p> | ||
| - | Ranaspumin 2 is one of six proteins used by the tungara frog (<i>Engystomops pustulosus</i>, found in Central and South America) to form protein foam nests in which it deposits its eggs. Protein foams are relatively rare in biology, and in this case offer a biocompatible solution to incubation and protection from mechanical destruction, as well as anti-microbial properties. | + | Ranaspumin-2 is one of six proteins used by the tungara frog (<i>Engystomops pustulosus</i>, found in Central and South America) to form protein foam nests in which it deposits its eggs. Protein foams are relatively rare in biology, and in this case offer a biocompatible solution to incubation and protection from mechanical destruction, as well as anti-microbial properties. |
</p> | </p> | ||
| Line 27: | Line 28: | ||
<p> | <p> | ||
| - | Coded for by the gene <i>rsn-2</i>, | + | Coded for by the gene <i>rsn-2</i>, Ranaspumin-2 (Rsn2) is a monomeric surfactant protein, with an atomic mass of 11kDa. It has a molecular weight of 13415.2 and is comprised of 117 amino acids. In aqueous solution it adopts a tightly folded globular shape, which is uncharacteristic of surfactant proteins. In this 3D arrangement Rsn2 consists of a single helix over a four-stranded sheet, as can be seen in the image below. </p> |
| - | <img src="http://farm7.static.flickr.com/6155/6167506290_3278200261_m.jpg" alt="Ribbon structure of | + | <img src="http://farm7.static.flickr.com/6155/6167506290_3278200261_m.jpg" alt="Ribbon structure of Ranaspumin-2"/> |
| - | <p> <font size="1" font color="grey"> Image 1: Ribbons structure of | + | <p> <font size="1" font color="grey"> Image 1: Ribbons structure of Ranaspumin-2 A: Frontal view B: 90 degree-rotation (Mackenzie, 2009) </font> </p> |
<p> | <p> | ||
This structure is highly unusual for a protein with surfactant properties, and appears to convey no significant amphiphilicity. It is therefore believed that Rsn2 undergoes a significant conformational change when at the air-water interface, in which it will expose hydrophobic groups to the air and hydrophilic groups to the water beneath. (Mackenzie, 2009) </p> | This structure is highly unusual for a protein with surfactant properties, and appears to convey no significant amphiphilicity. It is therefore believed that Rsn2 undergoes a significant conformational change when at the air-water interface, in which it will expose hydrophobic groups to the air and hydrophilic groups to the water beneath. (Mackenzie, 2009) </p> | ||
| - | <a href="https://2011.igem.org/Wiki/2011.igem.org/Team:Glasgow/Ranaspumin2_Sequence"> Click here</a> for | + | <a href="https://2011.igem.org/Wiki/2011.igem.org/Team:Glasgow/Ranaspumin2_Sequence"> Click here</a> for Ranaspumin-2 sequence. |
<h2>Function</h2> | <h2>Function</h2> | ||
| Line 45: | Line 46: | ||
<ul><p>Kennedy, M.W., <a href="http://www.gla.ac.uk/schools/lifesciences/staff/malcolmkennedy/malcolmkennedy/ | <ul><p>Kennedy, M.W., <a href="http://www.gla.ac.uk/schools/lifesciences/staff/malcolmkennedy/malcolmkennedy/ | ||
proteinsoffrogfoamnests/">Proteins of Frog Foam Nests.</a> Date Accessed: 20/09/2011</p></ul> | proteinsoffrogfoamnests/">Proteins of Frog Foam Nests.</a> Date Accessed: 20/09/2011</p></ul> | ||
| - | + | <ul><p>Cooper, A., <a href=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2954283>Biofoams and natural protein surfactants</a> Date Accessed: 24/08/1991</p></ul> | |
</html> | </html> | ||
Latest revision as of 03:46, 22 September 2011

Ranaspumin-2
Back to Parts
Ranaspumin-2 is one of six proteins used by the tungara frog (Engystomops pustulosus, found in Central and South America) to form protein foam nests in which it deposits its eggs. Protein foams are relatively rare in biology, and in this case offer a biocompatible solution to incubation and protection from mechanical destruction, as well as anti-microbial properties.

Protein foam nest from Trinidad 1 |

A protein foam nest containing newly hatched frogspawn 2 |

Pustulosus constructing a foam nest in the wild 2 |
Structure
Coded for by the gene rsn-2, Ranaspumin-2 (Rsn2) is a monomeric surfactant protein, with an atomic mass of 11kDa. It has a molecular weight of 13415.2 and is comprised of 117 amino acids. In aqueous solution it adopts a tightly folded globular shape, which is uncharacteristic of surfactant proteins. In this 3D arrangement Rsn2 consists of a single helix over a four-stranded sheet, as can be seen in the image below.
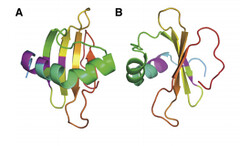
Image 1: Ribbons structure of Ranaspumin-2 A: Frontal view B: 90 degree-rotation (Mackenzie, 2009)
This structure is highly unusual for a protein with surfactant properties, and appears to convey no significant amphiphilicity. It is therefore believed that Rsn2 undergoes a significant conformational change when at the air-water interface, in which it will expose hydrophobic groups to the air and hydrophilic groups to the water beneath. (Mackenzie, 2009)
Click here for Ranaspumin-2 sequence.Function
The protein undergoes a conformational refolding whilst in solution, exhibiting significant surfactant properties at the air-water interface. This property makes it potentially useful when working with biofilms that form at the same phase interface, as it is thought that it may assist in the destruction of the biofilm.
References
Mackenzie et al., 2009. Ranaspumin-2: structure and function of a surfactant protein from the foam nests of a tropical frog. Biophysical Journal, 96, pp. 4984-4992
Kennedy, M.W., Proteins of Frog Foam Nests. Date Accessed: 20/09/2011
Cooper, A., Biofoams and natural protein surfactants Date Accessed: 24/08/1991
 "
"
