Team:Wageningen UR/Project/CompleteProject1Description
From 2011.igem.org
(→Synchroscillator) |
(→Synchroscillator) |
||
| (98 intermediate revisions not shown) | |||
| Line 25: | Line 25: | ||
{{:Team:Wageningen_UR/Templates/NavigationTop1}} | {{:Team:Wageningen_UR/Templates/NavigationTop1}} | ||
==Synchroscillator== | ==Synchroscillator== | ||
| - | {{:Team:Wageningen_UR/Templates/ | + | {{:Team:Wageningen_UR/Templates/NavigationLeftProjectDescription}} |
{{:Team:Wageningen_UR/Templates/Style | text= __NOTOC__ | {{:Team:Wageningen_UR/Templates/Style | text= __NOTOC__ | ||
| - | === | + | ===Project Description=== |
====1. Introduction==== | ====1. Introduction==== | ||
| - | The aim of this project is to design and implement a system exhibiting sustained oscillatory protein expression which is synchronized across a population of spatially constrained E. coli cells. The principles that govern this type of | + | The aim of this project is to design and implement a system exhibiting sustained oscillatory protein expression which is synchronized across a population of spatially constrained ''E. coli'' cells. The principles that govern this type of behavior have been studied both in theory and in practice, and as such there exists a solid foundation to apply these ideas in the context of the iGEM competition. In essence, this project consists of constructing a plasmid which contains protein encoding genes which reciprocally affect each other’s expression in a reliable manner, and experimentally measuring the expression dynamics to test the predictive value of a mathematical model. Due to the specificity of the required system parameters, and resulting difficulty in experimentally verifying the phenomena we wish to observe, special considerations regarding the experimental set-up had to be made. We hope that this system might be employed as a pace-making device to drive more complex genetic circuits requiring time-dependent gene expression, or as a component in sophisticated metabolic engineering applications. |
| Line 40: | Line 40: | ||
====2. Mechanism==== | ====2. Mechanism==== | ||
| - | There are a number of genetic circuit topologies that have the potential to exhibit oscillatory | + | There are a number of genetic circuit topologies that have the potential to exhibit oscillatory behavior under the right conditions. However, the requirement that the oscillations should be synchronized posed a constraint on the components that could be used. The starting point for our genetic circuitry was a design recently published in the article “A synchronized quorum of genetic clocks” by Danino et al. This design combines elements of the ''Vibrio fischeri'' quorum sensing system with a quorum quenching enzyme from ''Bacillus subtilis'', resulting in coupled positive and negative feedback loops which regulate the expression of a reporter protein. |
| + | '''Basic Components:''' | ||
| - | + | '''LuxR''' is a transcriptional regulator in the bioluminescent quorum-sensing system of the symbiotic deep sea bacterium ''Vibrio fischeri''. It is induced by binding the auto-inducer molecule N-(3-oxohexanoyl)-homoserine lactone (AHL). The AHL-LuxR complex controls expression of the lux regulon, which contains diverging pRight and pLeft promoter elements. The pRight element has low basal transcription, and is activated by AHL-LuxR; pLeft has higher basal expression, and is repressed by the AHL-LuxR complex. This dual activity makes LuxR a useful element for controlling interconnected genetic feedback loops. The unrestricted diffusion of AHL through the plasma membrane allows spatially proximate populations of cells to experience identical AHL conditions and synchronize AHL-dependent gene expression. | |
| - | |||
| - | ''' | + | The enzyme '''LuxI''' is an acyl-homoserine-lactone synthase which produces the intercellular signalling molecule N-(3-oxohexanoyl)-homoserine lactone (AHL). Placing LuxI under control of the pRight promoter results in a positive feedback loop: when increases in cell density cause the intracellular AHL concentration to rise above the activation threshold of the pRight promoter, the transcription rate of the LuxI gene is increased which in turn results in the production of more AHL. |
| - | |||
| - | + | '''AiiA''' is an enzyme from ''B. subtilis'' which degrades AHL. Its biological function is to interfere with the quorum sensing signals of other bacteria. Placing it under control of the pRight promoter results in negative feedback as a response to increasing AHL concentrations. | |
| - | |||
| - | |||
| - | |||
| + | The reporter molecule Green Fluorescent Protein ('''GFP''') is also regulated by the pRight promoter and provides a quantitative (albeit delayed) indication of the AHL concentration the cell is exposed to at a given point in time. | ||
| + | LuxI, AiiA and GFP are all tagged for rapid degradation (LVA-tag). Due to differences in the synthesis and degradation rates of LuxI and AiiA, there exists a space of conditions within which periodic oscillations in AHL concentration, and concomitant oscillatory protein expression can emerge. Under most conditions, the level of AHL within a population of cells will quickly reach a steady state. However, by simulating the system using a quantitative biochemical model, it is possible to predict conditions under which oscillations are likely to occur. See our [https://2011.igem.org/Team:Wageningen_UR/Project/ModelingProj1 modeling page] for details. | ||
| - | |||
| - | |||
| - | |||
| - | '''Fig. | + | |
| + | [[File:System1.png|760px|link=https://static.igem.org/mediawiki/2011/d/d0/System1.png]] | ||
| + | |||
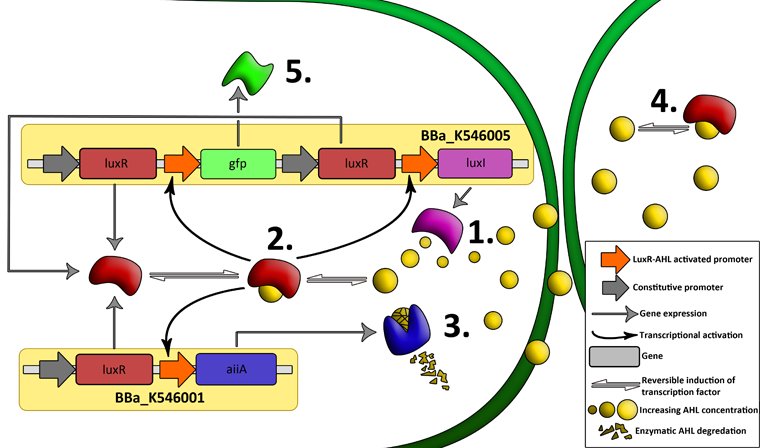
| + | '''Fig.2.''' ''Genetic circuit of a synchronized oscillator on 2 plasmids'' | ||
| + | |||
| + | '''1''': The AHL Synthase LuxI is expressed at a basal level when AHL concentration is low. Relative AHL concentration increases as a function of cell density. | ||
| + | |||
| + | '''2''': As the concentration of AHL increases, it associates with the constitutively expressed transcription factor LuxR, thereby up-regulating the expression of AiiA, LuxI and GFP. As LuxI accumulates, the concentration of AHL increases rapidly as a result of the LuxR-AHL -> LuxI positive feedback loop. | ||
| + | |||
| + | '''3''': The AHL-degrading enzyme AiiA does not accumulate as rapidly as LuxI. However, the degradation of AHL occurs at a faster rate than AHL synthesis, effectively reducing the level of active LuxR (and its transcriptional activity). This delayed negative feedback results in oscillations in AHL concentration. | ||
| + | |||
| + | '''4''': AHL diffuses freely between cells, synchronizing LuxR-AHL dependent transcriptional activity across a population of cells. | ||
| + | |||
| + | '''5''': LuxR-AHL dependent GFP expression allows the fluctuations in AHL concentration to be observed in situ. LuxI, AiiA and GFP all have LVA tags and are degraded rapidly (albeit at slightly different rates). | ||
| + | |||
| + | [[Team:Wageningen_UR/Project/CompleteProject1Description#Project Description| back to top]] | ||
| + | |||
| + | |||
| + | ====3. Designs==== | ||
| + | '''“Hasty” system:''' | ||
| + | |||
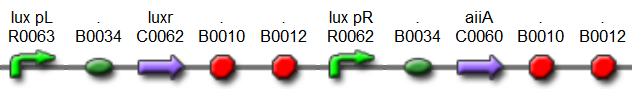
| - | The first design we implemented was a BioBrick part based reconstruction of the plasmids used by Danino | + | The first design we implemented was a BioBrick part based on reconstruction of the plasmids used by Danino et al. We intended to make as accurate a replica as possible in order to confirm the previously published results, and to test the viability of our experimental [https://2011.igem.org/Team:Wageningen_UR/Project/Devices platform]. However, there are a few differences between the original Hasty system and “our replica“. While the Hasty system employs the natural lux promoter which contains divergent pLeft and pRight elements (Fig 4), the BioBrick parts we employed have both elements in the same orientation. Both the original and our system contain 3 copies of the luxR gene under control of the pLeft element. Furthermore, a different (high copy) backbone was used during the functional validation of the parts, as opposed to the low copy backbone employed by Hasty. |
| + | |||
[[File:Hasty1.png|x67px|link=http://partsregistry.org/Part:BBa_K546005]] | [[File:Hasty1.png|x67px|link=http://partsregistry.org/Part:BBa_K546005]] | ||
| - | '''Fig. | + | |
| + | '''Fig.4.''' ''BioBrick part K546005 forms the positive feedback loop component of the basic synchonized oscillator. | ||
| + | |||
[[File:Hasty2.png|x67px|link=http://partsregistry.org/Part:BBa_K546001]] | [[File:Hasty2.png|x67px|link=http://partsregistry.org/Part:BBa_K546001]] | ||
| - | '''Fig. | + | '''Fig.5.''' ''BioBrick part K546001 forms the negative feedback loop component and complements K546005.'' |
| + | Due to the tendency of interconnected positive and negative feedback loops to reach a steady state rather than sustaining oscillations, the external conditions need to be precisely controlled in order for the system to produce synchronized oscillations. In this system, the only parameters relevant to oscillatory gene expression that can be controlled during operation are the cell density and the external AHL degradation rate (see [https://2011.igem.org/Team:Wageningen_UR/Project/ModelingProj1 model] for details). | ||
| + | However, maintaining a constant cell density over extended periods of time while independently varying the flow rate (AHL degradation) is difficult to achieve using traditional cultivation and measurement set-ups. In order to control these parameters while keeping the cells microscope-accessible, we developed a custom fluidic platform that can trap cells and allow them to remain viable over the duration of an experiment while maintaining a constant and reproducible cell density (See [https://2011.igem.org/Team:Wageningen_UR/Project/Devices flow chamber page] for details). | ||
| - | |||
| - | |||
| - | + | '''Double Tunable Synchronized Oscillator''' | |
| - | + | The aforementioned constraints are due to the fact that there are no means by which the expression kinetics of LuxI and AiiA can be externally influenced. In order to gain more control over the expression dynamics, we refactored the system and introduced “tuning knobs” in the form of chemically inducible hybrid promoters (pR/LacI inducible by IPTG and pR/tetR inducible by anhydrotetracycline). The addition of two independent control variables should substantially expand the parameter space (cell density, flow rate) within which oscillations can occur. We also made the design more streamlined by taking advantage of the great diversity of available BioBrick quorum sensing parts in order to remove redundant elements and fit the entire system onto a single plasmid (a 30% reduction in size compared to the original design). Note: in order to take advantage of the tuning capabilities it is necessary to co-transform a plasmid which constitutively expresses LacI or TetR, or both. | |
| - | [[File: | + | [[File:System2.png|760px|link=https://static.igem.org/mediawiki/2011/a/a8/System2.png]] |
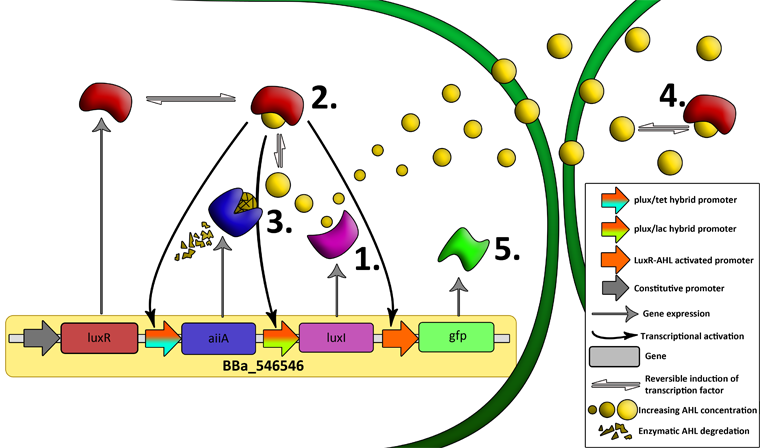
| - | '''Fig.6.''' '' | + | '''Fig.6.''' ''Genetic circuit of the synchronized oscillating system on a single plasmid.'' |
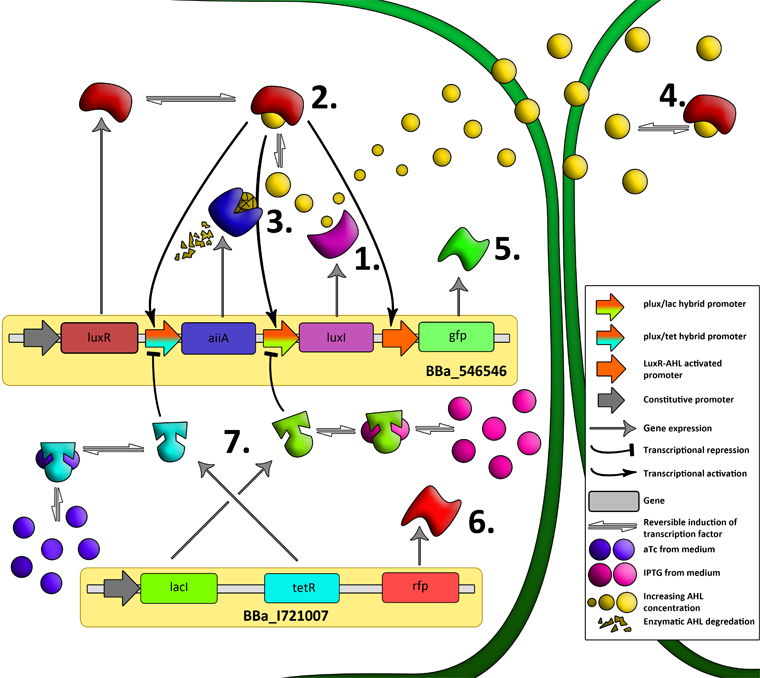
| - | = | + | [[File:System3.png|760px|link=https://static.igem.org/mediawiki/2011/e/e1/System3.png]] |
| - | + | ||
| + | '''Fig.7.''' ''Genetic circuit of a tunable synchronized oscillator.'' | ||
| - | |||
| - | ''' | + | '''1''': The AHL Synthase LuxI is expressed at a basal level when AHL concentration is low. Relative AHL concentration increases as a function of cell density. |
| + | '''2''': As the concentration of AHL increases, it associates with the constitutively expressed transcription factor LuxR, thereby up-regulating the expression of AiiA, LuxI and GFP. As LuxI accumulates, the concentration of AHL increases rapidly as a result of the LuxR-AHL -> LuxI positive feedback loop. | ||
| - | + | '''3''': The AHL-degrading enzyme AiiA does not accumulate as rapidly as LuxI. However, the degradation of AHL occurs at a faster rate than AHL synthesis, effectively reducing the level of active LuxR (and its transcriptional activity). This delayed negative feedback results in oscillations in AHL concentration. | |
| - | ''' | + | '''4''': AHL diffuses freely between cells, synchronizing LuxR-AHL dependent transcriptional activity across a population of cells. |
| + | '''5''': LuxR-AHL dependent GFP expression allows the fluctuations in AHL concentration to be observed in situ. LuxI, AiiA and GFP all have LVA tags and are degraded rapidly (albeit at slightly different rates). | ||
| - | + | '''6''': Constitutively expressed RFP allows the distinction between the effects of cell growth and of the true oscillations of GFP expression due to our system. | |
| - | + | ||
| + | '''7''': The repressors LacI and TetR are constitutively expressed. They inhibit the expression of luxI and aiiA respectively. Adding IPTG removes the LacI repressor and aTc removes the TetR repressor. | ||
| - | [[File: | + | [[File:Dt.png|x67px|link=http://partsregistry.org/Part:BBa_K546546]] |
| - | '''Fig. | + | '''Fig.8.''' ''The complete synchronized oscillator circuit with a single copy of constitutively expressed LuxR.'' |
| - | |||
| - | + | [[Team:Wageningen_UR/Project/CompleteProject1Description#Project Description| back to top]] | |
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | ==== | + | ====4. Experimental verification==== |
| - | + | ||
| - | + | The individual subcomponents of the oscillatory circuit were tested using a fluorescence spectrophotometer. Changes in GFP expression relative to different AHL concentrations were measured to verify the functionality of the parts (see [https://2011.igem.org/Team:Wageningen_UR/Project/PartsProj1 data page]). However, due to the precise growth conditions required for synchronized oscillations to be sustained, it was necessary to adopt a more sophisticated approach to test the complete system. | |
| - | + | ||
| - | + | There were three requirements for the experimental set-up to test the Synchronized Oscillatory System: | |
| - | + | ||
| + | a) ability to grow cells in a physically constrained space to achieve the high cell density necessary for quorum sensing machinery to respond. Ideally, the cell density should remain more or less constant over the course of hours, in addition to being predictable and reproducible. | ||
| + | |||
| + | b) supplying cells with fresh media to provide nutrients and remove waste (and AHL) without affecting the cell density. | ||
| + | |||
| + | c) directly observe cells with a fluorescence microscope to perform time-lapse studies aiming at detecting changes in GFP expression over a period of hours. | ||
| + | |||
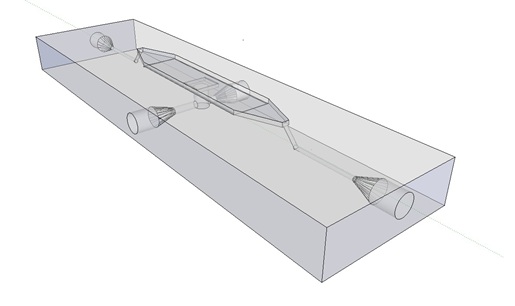
| + | Previous studies of oscillatory gene expression have employed microfluidic devices with micron-scale trapping chambers to restrict cells. However, these systems can be prohibitively expensive, require specialized accessories, and are generally not reuseable. We developed a low-cost experimental flow chamber that fulfills all of the aforementioned criteria (for detailed specifications see [https://2011.igem.org/Team:Wageningen_UR/Project/Devices flow chamber page]) . | ||
| + | |||
| + | |||
| + | [[File:Mainpic.jpg]] | ||
| + | |||
| + | '''Fig.9.''' ''3D rendering of the flow chamber.'' | ||
| + | |||
| + | |||
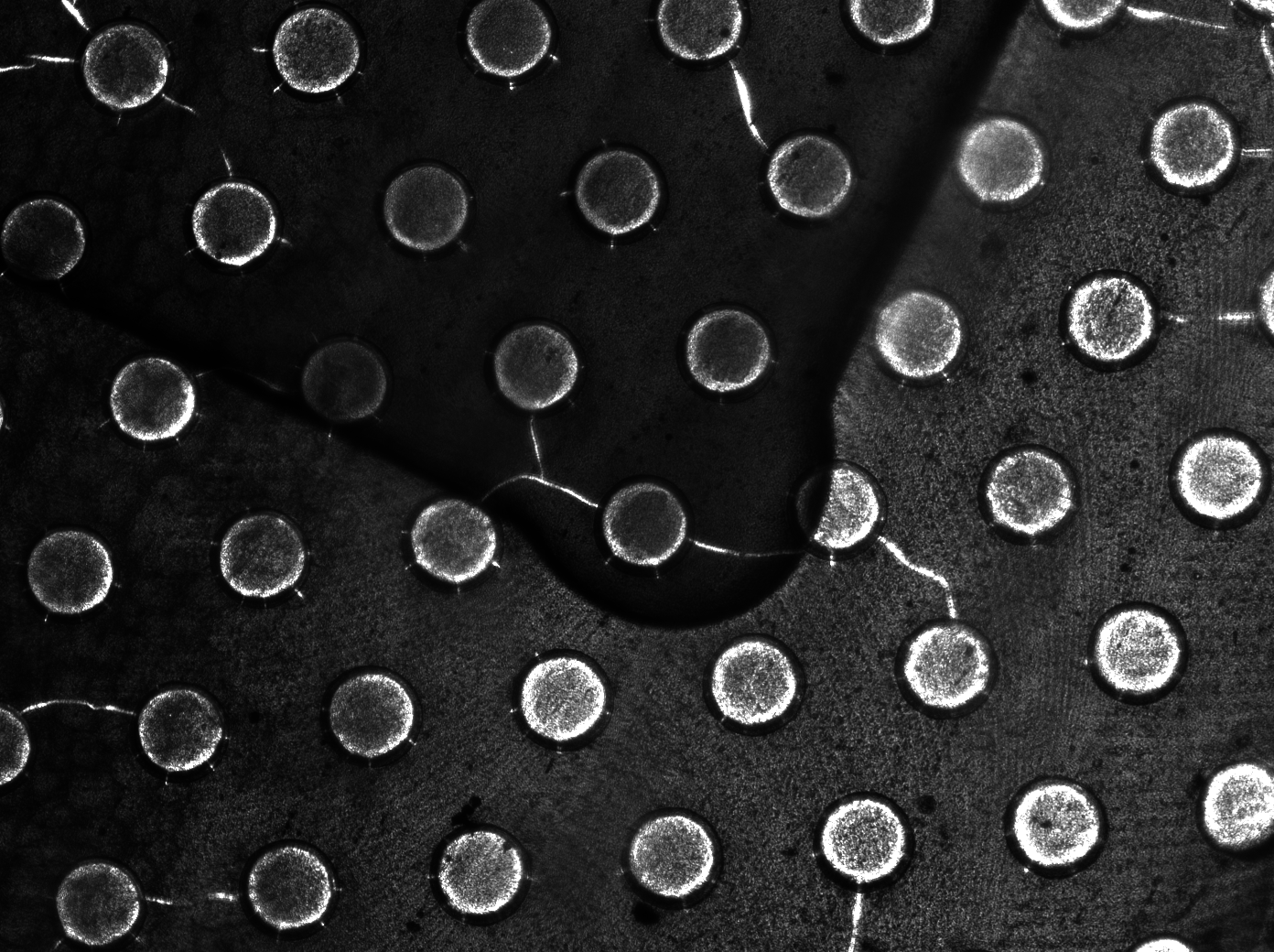
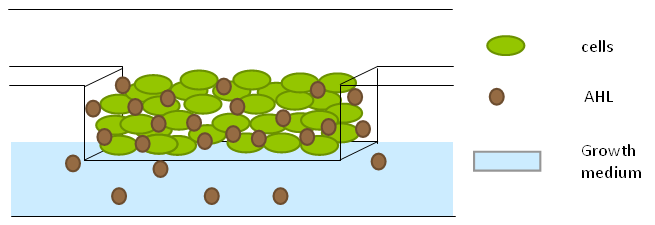
| + | To measure the oscillations, the flow chamber was outfitted with a microdish, which is a thin aluminium oxide sheet containing thousands of circular wells. The wells are 180 μm in diameter and 40 μm deep. Small molecules (such as nutrients from growth media) can diffuse through the bottom of the dish and feed cells growing in the wells. For these experiments, the cells were fed through bottom in order to restrict their growth to the wells. This allowed the cells to reach high densities and relative stable populations which could be studied individually (4 wells could be visualized using 100x magnification) | ||
| + | |||
| + | |||
| + | [[File:Dish.gif]] [[File:Microdish_close.PNG|400px|link=https://static.igem.org/mediawiki/2011/3/3e/Microdish_close.PNG|right]] | ||
| + | '''Fig.10.''' ''Above: Schematic illustration of the microdish.'' | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | '''Fig.11.''' ''Right: Microdish installed in flow chamber viewed at 40x magnification, illuminated from the bottom with white light. Growth medium is supplied from the bottom through the 5x5mm socket (rounded corner). Diffusion of nutrients through the aluminium oxide allows cells to grow across the entire dish. | ||
| + | |||
| + | |||
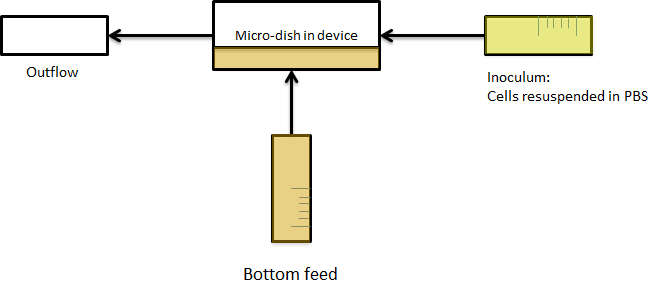
| + | The cells containing the synchronized oscillator system plasmid were grown overnight in LB medium supplemented with an antibiotic. They were then centrifuged for 10 minutes at 4600 rcf and resuspended in Phosphate Buffered Saline solution before being injected through the top flow port (Fig.12a). Directly after injection, the cells fill the entire chamber volume (Fig.13a). After approximately 5-8 hours the cells situated outside the wells are starved and no longer express detectable levels of GFP (Fig.13b). Cells in the wells continue to grow for approximately 12 hours before reaching a stable cell density (Fig.13c). After the wells were saturated with cells, time-lapse microscopy was initiated. A photograph was taken every 10 minutes using a fluorescence microscope fitted with a GFP filter. Changes in GFP expression over time were subsequently quantified. (Video 1) | ||
| + | |||
| + | |||
| + | [[File:Bottom_feed_WUR.png|380px]] [[File:Zero-flow.PNG|380px]] | ||
| + | |||
| + | '''Fig.12a.''' ''Schematic view of the bottom fed flow device.'' '''Fig.12b.''' ''Three dimensional impression of cells in the flow device'' | ||
| + | |||
| + | |||
| + | [[File:Fullchamber.PNG|250px]] [[File:Dish_cells.JPG|250px]] [[File:4pbs_experiment_incubatenewof17.8_DT_5g_dry.jpg|250px]] | ||
| + | |||
| + | '''Fig.13a.''' ''Cells in the chamber, directly after injection.'' '''Fig.13b.''' ''Cells in the chamber, a few hours after injection.'' '''Fig.13c.''' ''Cells in the chamber, about twelve hours after injection.'' | ||
| + | |||
| + | |||
| + | |||
| + | ====5. Results==== | ||
| + | |||
| + | |||
| + | Per run four wells of the microdish were analyzed, as this is the maximal number of wells that one can focus on with a 10x objective. Pictures were processed with [http://rsbweb.nih.gov/ij/ ImageJ], a program that can measure the different wells separately. | ||
| + | |||
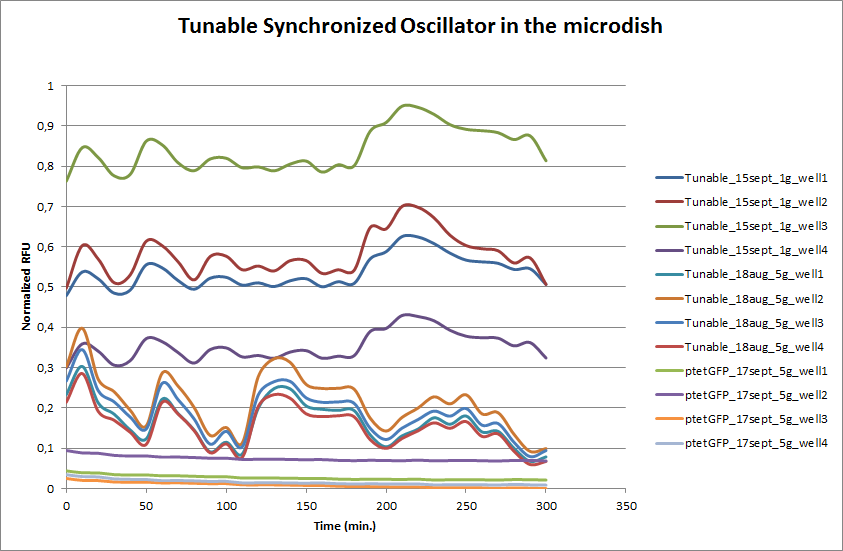
| + | These time-lapse microscopy studies of cells harboring the Synchroscillator plasmid consistently showed significant periodic changes in GFP expression compared to control experiments with cells constitutively expressing GFP (Fig.15). The observed periodicity is in the range of 60 minutes, and the asymmetry of the peaks is indicative of differences between GFP synthesis and degradation. The oscillations were sustained over multiple periods for a duration of 5 hours after which the expression level of GFP decreased; most likely due to decreases in cell vitality. These findings are consistent with predictions made from mathematical simulations as well as previously published results. It is noteworthy that these dynamics were able to emerge under zero-flow conditions and did not require manipulation the external AHL degradation rate. The synchronization between wells suggests that the diffusion of extracellular AHL through the bottom of the microdish occurred at a rate that allowed spatially separated cell populations to experience similar AHL concentrations. | ||
| + | |||
| + | |||
| + | [[File:Tunabledata.png|760px]] | ||
| + | |||
| + | '''Fig.14''' ''Quantitative analysis of GFP expression by the Tunable Oscillator in the microdish. Negative control: ptet-GFP (fluorescent, non-oscillating). | ||
| + | |||
| + | <html><iframe width="420" height="315" src="http://www.youtube.com/embed/Q6tDQNIaklg" frameborder="0" allowfullscreen></iframe></html> | ||
| + | |||
| + | '''Fig.15''' ''Time-lapse video of the Tunable Oscillator.'' | ||
| + | |||
| + | ====6. Conclusion and Future work==== | ||
| + | |||
| + | Over the course of our experiments we were able to show that quorum sensing genes and regulatory elements composed to form interconnected positive and negative feedback loops can result in sustained synchronized oscillations under appropriate experimental conditions. | ||
| + | |||
| + | These findings highlight the utility of custom designed micro-environments for the study of complex genetic circuits. Our flow chamber outfitted with the microdish proved to be a suitable platform to investigate the temporal dynamics of intercellular communication. The ability to manipulate extracellular conditions without disrupting localized cell populations should prove useful in investigating biological systems in real time. | ||
| + | |||
| + | Future work should focus on defining appropriate wet-lab experiments in order to enhance the quality of the mathematical model by fitting it with the right parameters. Also the stability of the oscillations can be improved by optimizing the supply of nutrients to the bacteria in the wells in order to achieve long term viability of the cell cultures. Additionally, the tunability of the system could enhance the robustness of such synthetic regulatory systems. Modularity BioBrick parts expand functionality without having to redesign the system from the ground up. | ||
| + | |||
| + | ===Links and references=== | ||
| + | |||
| + | [1][http://www.nature.com/nature/journal/v463/n7279/abs/nature08753.html Danino et al. 2010] | ||
| + | |||
| + | [2][http://www.nature.com/nature/journal/v463/n7279/suppinfo/nature08753.html Supplementary information] | ||
| + | |||
| + | [3][https://2007.igem.org/Tokyo/Works Tokyo iGEM 2007] | ||
| + | |||
| + | [4][http://rsif.royalsocietypublishing.org/content/early/2010/06/28/rsif.2010.0183.short A comparative analysis of synthetic genetic oscillators] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | [[Team:Wageningen_UR/Project/CompleteProject1Description#Project Description| back to top]] | ||
| + | |||
}} | }} | ||
Latest revision as of 03:53, 22 September 2011
 "
"