Team:Cambridge/Experiments/Reflectin Thin Films II
From 2011.igem.org
(→Results) |
|||
| Line 1: | Line 1: | ||
| - | {{Template:Team:Cambridge/ | + | {{Template:Team:Cambridge/CAM_2011_EXPERIMENT_HEAD}} |
=Reflectin Thin Films II= | =Reflectin Thin Films II= | ||
Thin films were produced after refining our earlier methods described in the experiment [https://2011.igem.org/Team:Cambridge/Experiments/Reflectin_Thin_Films_I reflectin thin films I], during which we discovered some very interesting properties. | Thin films were produced after refining our earlier methods described in the experiment [https://2011.igem.org/Team:Cambridge/Experiments/Reflectin_Thin_Films_I reflectin thin films I], during which we discovered some very interesting properties. | ||
| Line 91: | Line 91: | ||
The new hazards identified with the changes are the liquid CO2 pressure washer and the hot plate. Dr Matthew Hawkeye performed all use with the pressure washer in the fume hood, whilst care was taken with the hot plate, using tweezers and wearing gloves to manipulate the hot substrates and allowing time to cool prior to mounting for microscopy. | The new hazards identified with the changes are the liquid CO2 pressure washer and the hot plate. Dr Matthew Hawkeye performed all use with the pressure washer in the fume hood, whilst care was taken with the hot plate, using tweezers and wearing gloves to manipulate the hot substrates and allowing time to cool prior to mounting for microscopy. | ||
| - | {{Template:Team:Cambridge/ | + | {{Template:Team:Cambridge/CAM_2011_EXPERIMENT_FOOT}} |
Latest revision as of 20:12, 21 September 2011
Contents |
Reflectin Thin Films II
Thin films were produced after refining our earlier methods described in the experiment reflectin thin films I, during which we discovered some very interesting properties.
Methods
During this second day we aimed to further explore some more properties of reflectin and the limitations of our equipment:
- Centrifugation of the solutions prior to coating to sediment insoluble impurities (13000rpm for 10 minutes). This is hoped to reduce the level of solid impurities, but preserve the HFIP soluble Reflectin in solution.
- Introduction of liquid CO2 pressure wash for cleaning of silicon substrates. O2 plasma may have changed the surface chemistry of the silicon. It is hoped physical cleaning will provide a better interface for subsequent coating.
- Trialled spin coating on glass substrate to investigate the effect of different substrates and obtain microscope images of the thin films illuminated from above and below.
- Heat-cured the thin films for varying amounts from couple of seconds to 30 minutes at 80oc to assess the impact of temperature
- Measured the reflectance spectra of some of the thin films to check colouration
- BSA (Bovine Serum Albumin) control to assess generic properties of proteinaceous thin films subject to the same coating conditions.
Results
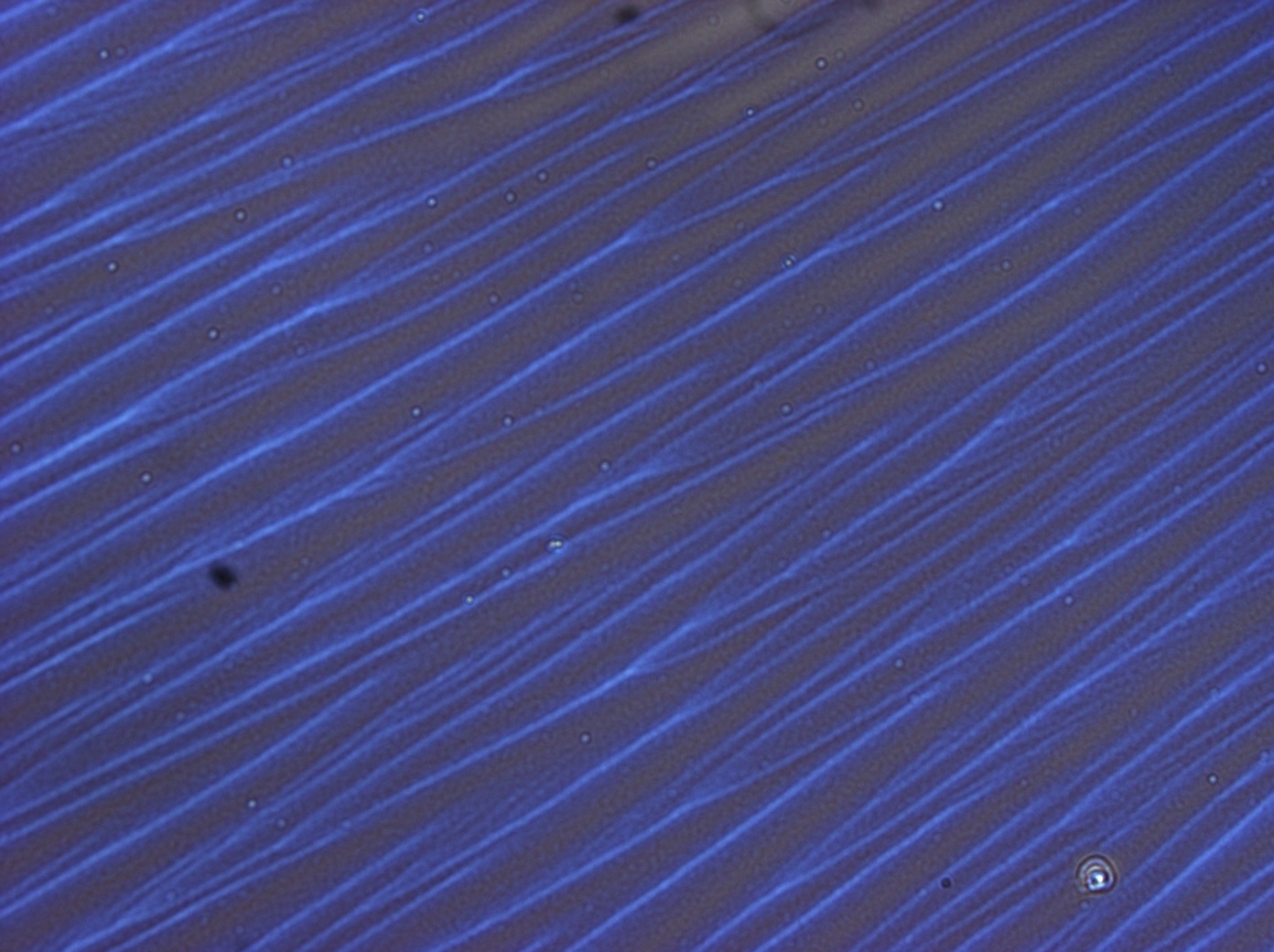
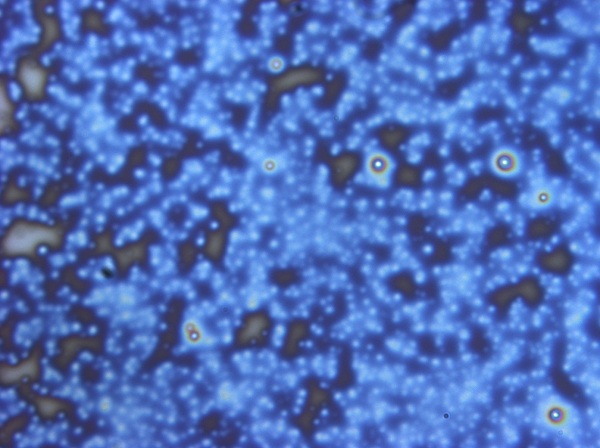
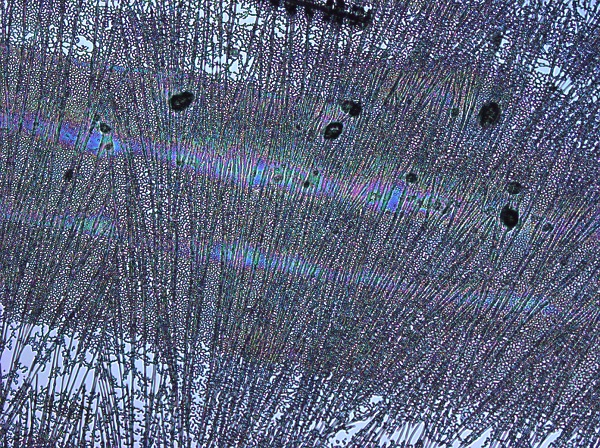
The result of centrifugation has led to dramatic differences in the spin-coated films. Below are shown typical results. The first image highlights the films produced on the first day whilst the second image highlights films produced using the refined protocol, finally the third image shows the BSA control produced using the refined protocol
This film shows mainly dark spots suggesting diffuse scattering and therefore solid impurities and great variations in colour, which implies a variation in film thickness and confirmed when we imaged them under the confocal microscope.
This film produced from the refined protocol demonstrates a much more uniform colour, dark blue in the bottom left fading to yellow in the top right, an observation which correlates with obtained spectral data. There are still numerous marks that suggest impurities or the possibility the films are not aggregating properly, but they are dominated by smooth blue and yellow regions.
The BSA control shows the films formed do not seem to demonstrate the same 'iridescence' as reflectin, though it does reflect a very discrete selection of wavelengths, mainly blue as opposed to the multicolours of reflectin films. There are however much less disruption in the form of impurities, most likely as the bovine serum albumin was purified industrially as opposed to the small scale methods we are implementing in the lab.
Further Observations and Conjecture
Investigating the Source of the Impurities in the Thin Films
- The HFIP solvent was centrifuged but no pellet/sediment was observed and the resulting spin-coat left only silicon substrate
- The resulting pellet from reflectin-HFIP centrifugation was re-suspended in HFIP. It was found to be mainly insoluble and subsequent coating did not yield a film with interference effects.
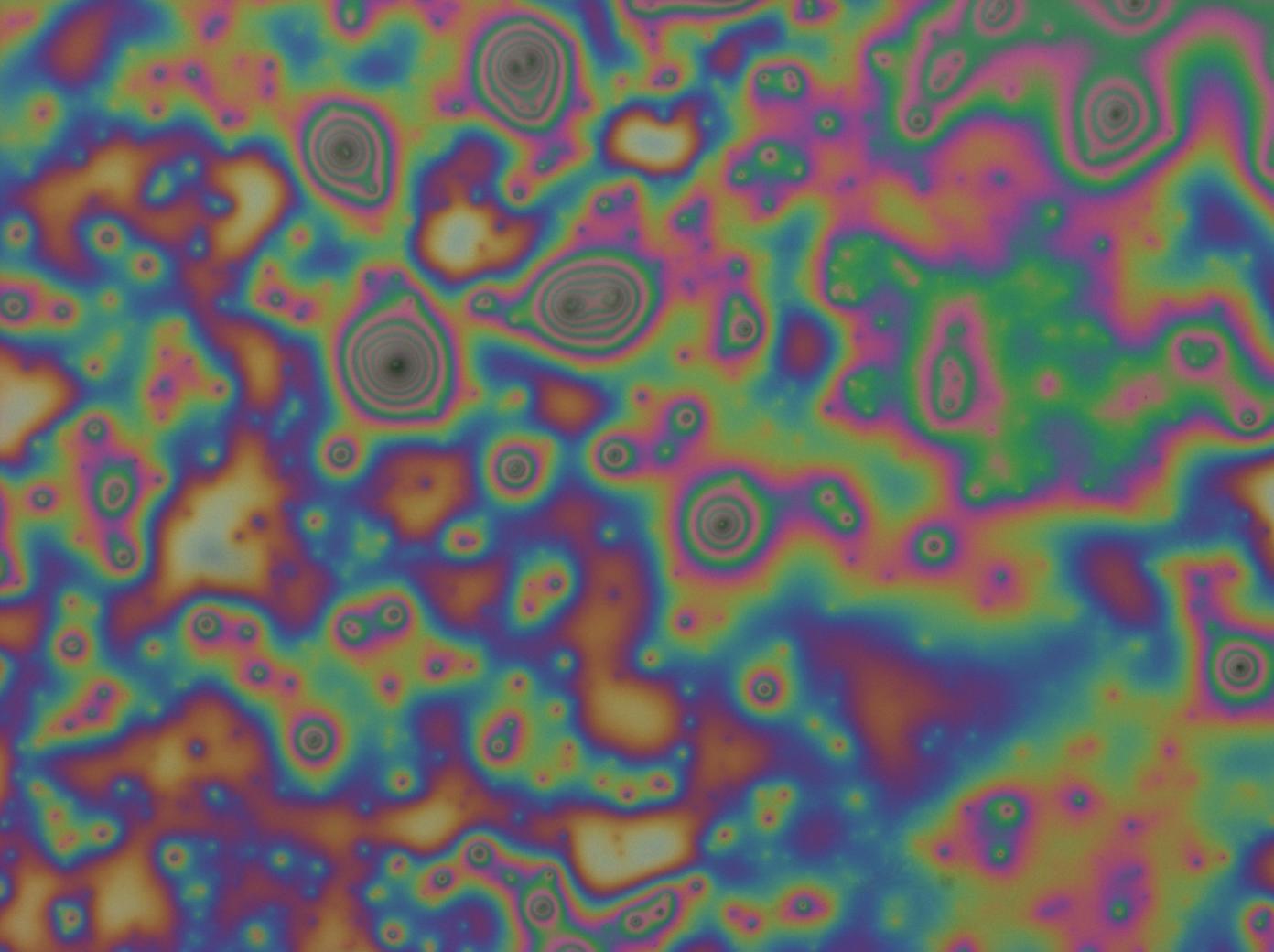
- The supernatant from reflectin-HFIP centrifugation was spin-coated and though the films were more uniform in colour careful examination yielded areas of film dissociation and black spots surrounded by interference rings much akin to newton's rings.
It is thus conjectured based on these observations that the purity of reflectin affects the quality of film produced and there may be soluble as well as insoluble contaminants, the source of which probably lies within the extracted protein.
Crystallization Behaviour
Whilst making the thin films the spontaneous formation of branch-like structures was first observed when the colour of the thin films spontaneously seemed to 'peel' off as we picked up the coated substrate with tweezers. On closer inspection under the optical microscope, a network of needle-like structures had formed from the films on the substrate. Below is a collection of observations concerning the conditions when these structures formed and some of its properties.
Conditions for Spontaneous Self Assembly of Needle-like Structures
The sample films were formed under different conditions with good samples repeated, for each different condition some were spin-coated and heat-treated/non heat-treated and some flow-coated and heat treated/non-heat treated. Reflectin films were made with 50μl of 10% weight by weight reflectin-HFIP solution.
- It was found the majority of reflectin films were unstable. For some reason the films spontaneously crystallized. The process seemingly stripped the films of its colour and left a colourless branching array of needle-like crystalline structures which seemed to be made from the thin film. This was true of both flow-coated and spin-coated films
- All of the spindle-like structures seemed to nucleate at points where tweezers were used to pick them up and seemed to require this as a stimulus. Whilst with some this occurred the first time handling it with a tweezer with others this only occurred after a period of time.
- The crystallization process seemed to depend on the size of the substrate. On large(>4cm2) and small(<1cm2) substrates crystals formed much more quickly than medium sized substrates. Time lengths varied from immediately after coating to 1-2 days afterwards to never.
- One of the films which was heated at 80oc for 30 mins immediately after spin-coating on a medium sized substrate 3000rpm for 30secs, no nucleation was ever observed no matter how it was tampered with. This suggests heat treatment inhibits crystallisation possibly by the evaporation of the solvent or promotion of cross-linking.
Final structure after Crystallization
- Different coating conditions and substrates produced spindle-like structures of different orientations and density. In one instance the structures formed were so dense it induced a band of iridescence orthogonal to spindle structures. Closer inspection revealed an intricate, regular inter-linked network of solution trapped between the spindles.
- It was found possible to induce further assembly of the needle like structures through poking of the substrate to provide vibrational energy. Under the microscope the solution first seemingly expands before forming the crystal-like spindles.
Reversible Crystallization The following experiments were carried out in order and the observations after each is documented.
- Effect of water droplets - On application of a droplet D.I water onto the thin films a dynamic play resulted and competition ensured between formation of the spindle-like structure and the 'iridescent' thin film. As the water droplet evaporated off the film the spindle-like structure became more energetically favourable.
- Effect of hydrolysing entire substrate - If the entire surface is re-hydrolysed, after evaporation a more intense iridescent thin film is recovered.
- Effect of re-heating substrate - Upon re-heating at 80oc reformation of the spindle-like structures occurs but less readily and initiated from nucleation points different from originally, almost as if it is nucleating around particular surface defects.
- Effect of water droplet after re-heating - After heat treatment, dropping water onto the film seems to destroy the underlying film, exposing the underlying silicon substrate. This did not occur if the film was rehydrolysed with water vapour by breathing on it nor did it occur with water droplets prior to heating.
Subjection of Films to Vapour
- Subjecting the film to water and ethanol vapour elicited a colour shift from blue to red in the EM spectrum. This was carried out using only the one sample where it retained its iridescence. This observation is in-line with thin-film interference and literature. The vapours induce swelling which leads to subsequent phase shifts in reflected wavelengths which exhibits itself as a colour shift.
Summary
- The BSA control illustrates proteins being themselves polymers can be cast into thin films with differing physical properties therefore conclusions made with reflectin may equally apply to other proteins. As the dominant composition of reflectin are the unusually high amounts of sulphur and aromatic compounds it is advisable to perform controls with similarly unusual proteins of similar composition.
- Self-assembly crystallization behaviour is due to dehydration of films, subsequent application of water seemed to reverse the change whilst heating restored the crystallization albeit at a much slower speed than initially. This spontaneous phase separation requires further study in particular reproduction of the conditions under which it forms initially after spin/flow coating. However subsequent prolonged 'curing' seemingly stabilises the film. The crystallization may also be due to other factors like the amount of reflectin or presence of contaminants. Further investigations are required.
- Colour changes can be initiated by application of water and ethanol vapours consistent with thin-film interference and existing literature.
Proposed Routes for Further Investigation
Further investigations based on our preliminary results can be split into the following avenues:
- Colour Control - The application of vapour to control colouration changes has been confirmed. A natural progression is to replicate more closely the natural colouration mechanism in squid due to phosphorylation. We propose to investigate further by submerging films in bath of phosphate donors e.g phosphoric acid and electrical control via formation of an electrical 'cell'. Our ultimate aim is to the production of an electronically controlled pixel.
- Colour Intensity - In close tandem with the above point, at present the coloured films are not very bright, the formation of multi-layer reflectin-polymer layers is expected to improve the intensity of colours and reproduce more closely the observed 'bragg stacks' formed natively in squid iridophores. Though we have initially experimented with coating reflectin atop a flexible polymer, PDMS no serious experimentation were undertaken. It is proposed to take this to the next level with the aim of producing similar thickness layers via spin coating and increasing layers using a 'swiss roll' technique where a two-layer film is rolled to produce multi-layers.
- Uniformity - To gain physical measures of uniformity of thicknesses of films we propose to image the produced thin films using AFM to build a topographic picture.
- Self-assembling Crystallizing behaviour - our purified proteins are not 100% pure, along with traces of buffers used in the his-tag purification process there are also the tris and triton remnants from vacuum centrifugation. Triton is a detergent and tris is known to crystallize together there is the worry they may be responsible for our observations as opposed to the reflection protein. This worry is strengthened by the purity of this first batch of proteins. It is therefore proposed to carry out further controls with buffers used in the purification process, tris and triton as well as repeating the experiments with a second batch of we believe (higher quality) reflectin proteins.
- Phase-separation - reflectin proteins have been documented in the literature to exhibit phase separation behaviour. Using BMIM as the solvent, reflectin phase separates into diffraction grating with spacings of 8-18μm upon dipping the formed thin-films in water therefore phase separation in HFIP is not unthinkable. It is proposed to investigate further reproduce this self-assembly behaviour in BMIM and investigate the tunability of the grating spacings after formation as well as aiming to reproduce the conditions under which the onset of crystallization was observed in reflectin-HFIP films.
Safety
The new hazards identified with the changes are the liquid CO2 pressure washer and the hot plate. Dr Matthew Hawkeye performed all use with the pressure washer in the fume hood, whilst care was taken with the hot plate, using tweezers and wearing gloves to manipulate the hot substrates and allowing time to cool prior to mounting for microscopy.
Back to Experiments
 "
"