Team:Freiburg/Notebook/19 July
From 2011.igem.org
(→Theoretical Gibson-Assembly) |
(→Precipitator) |
||
| Line 41: | Line 41: | ||
===NAME OF YOUR EXPERIMENT=== | ===NAME OF YOUR EXPERIMENT=== | ||
| + | '''PCR''' | ||
| - | + | ||
| + | {| style="border-spacing:0;" | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Name: Ruediger | ||
| + | |||
| + | |||
| + | |||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Date: 19.07.11 | ||
| + | |||
| + | |- | ||
| + | | colspan="2" style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Continue from Experiment (Date) | ||
| + | |||
| + | PCR 18.07 (Name) | ||
| + | |||
| + | |- | ||
| + | | colspan="2" style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Project Name: GFP Pbd | ||
| + | |||
| + | |} | ||
| + | PCR-Mixture for one Reaction: | ||
| + | |||
| + | For a 50 µl reaction use | ||
| + | |||
| + | |||
| + | {| style="border-spacing:0;" | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 32,5µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| H<sub>2</sub>0 | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Name | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 10µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 5x Phusion Buffer | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| of Primer | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 2.5µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Primer fw | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| P18, P19, P20 | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 2.5µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Primer dw | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| P28 | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 1µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| dNTPs | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| of Template DNA | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 1µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| DNA-Template | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| PCR product of P1,P3,S14 from yesterday | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 0.5 µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Phusion (add in the end) | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |} | ||
| + | What program do you use? | ||
| + | |||
| + | One set of probes was prepared and then split into 3 tubes each to test them at Annealingtemperatures 44C, 52C and 60C for the first 10 cycles. Then Annealingtemperature 60C for 25 cycles | ||
| + | |||
| + | |||
| + | To confirm the PCR-Product has the correct size, load 2 µl of the sample onto an agarose-gel. | ||
| + | |||
| + | |||
| + | How did you label the PCR-Product, where is it stored and what do you do next? | ||
| + | |||
| + | S14+P18+P28 | ||
| + | |||
| + | S14+P19+P28 | ||
| + | |||
| + | S14+P20+P28 | ||
| + | |||
| + | Stored in PCR product box | ||
| + | |||
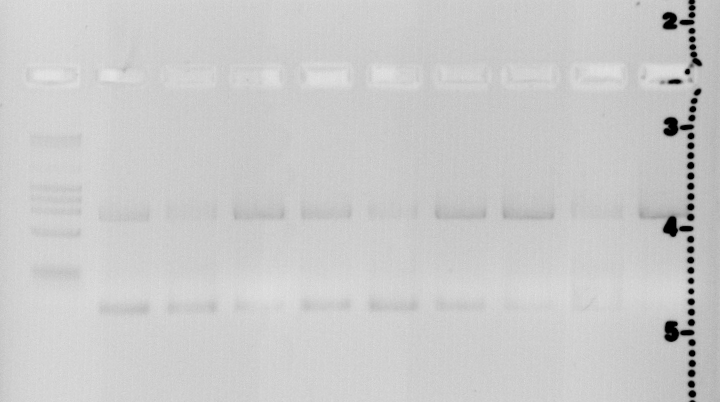
| + | [[File:Freiburg11_7_20_2011_2_48_01_PM.Jpg]] | ||
| + | Lane1 | ||
| + | Quick Load Marker | ||
| + | Lane2 | ||
| + | 44C S14+P18+P28 | ||
| + | Lane3 | ||
| + | 44C S14+P19+P28 | ||
| + | Lane4 | ||
| + | 44C S14+P20+P28 | ||
| + | Lane5 | ||
| + | 52C S14+P18+P28 | ||
| + | Lane6 | ||
| + | 52C S14+P19+P28 | ||
| + | Lane7 | ||
| + | 52C S14+P20+P28 | ||
| + | Lane8 | ||
| + | 60 S14+P18+P28 | ||
| + | Lane9 | ||
| + | 60 S14+P19+P28 | ||
| + | Lane10 | ||
| + | 60 S14+P20+P28 | ||
Revision as of 16:32, 19 July 2011
Contents |
green light receptor
NAME OF YOUR EXPERIMENT
Investigators:NAME
blue light receptor
Theoretical Gibson-Assembly
Investigators: Sandra, Sophie
Primer Degin for Blue light sensor (lovTAP) + trp promotor (BBa_K322999) and tetR Gen + tetO promotor (BBa_Q04400).
- LOVtap-Trp_up: gaattcgcggccgcttctagtcacacaggaaagtactatgt
- LOVtap-Trp_dw: tacttttatctaatctggacatctagtatttctcctctttgtcgataccctttttacgtg
- TetR-TetO_up: aaagaggagaaatactagatgtccagattag
- TetR-TetO_dw^: ctgcagcggccgctactag
^we also have another primer for this one, but we are not sure about the length/melting temperature. Might be changed.
red light receptor
NAME OF YOUR EXPERIMENT
Investigators:NAME
Lysis cassette
NAME OF YOUR EXPERIMENT
Investigators:NAME
Precipitator
NAME OF YOUR EXPERIMENT
PCR
| Name: Ruediger
| Date: 19.07.11 |
| Continue from Experiment (Date)
PCR 18.07 (Name) | |
| Project Name: GFP Pbd | |
PCR-Mixture for one Reaction:
For a 50 µl reaction use
| 32,5µl | H20 | Name |
| 10µl | 5x Phusion Buffer | of Primer |
| 2.5µl | Primer fw | P18, P19, P20 |
| 2.5µl | Primer dw | P28 |
| 1µl | dNTPs | of Template DNA |
| 1µl | DNA-Template | PCR product of P1,P3,S14 from yesterday |
| 0.5 µl | Phusion (add in the end) |
What program do you use?
One set of probes was prepared and then split into 3 tubes each to test them at Annealingtemperatures 44C, 52C and 60C for the first 10 cycles. Then Annealingtemperature 60C for 25 cycles
To confirm the PCR-Product has the correct size, load 2 µl of the sample onto an agarose-gel.
How did you label the PCR-Product, where is it stored and what do you do next?
S14+P18+P28
S14+P19+P28
S14+P20+P28
Stored in PCR product box
 "
"