July, 4th
Digestions of previously purified plasmids were performed for ligations:
| Plasmid |
Kind |
DNA (μl) |
H2O (μl) |
Enzyme 1 (μl) |
Enzyme 2 (μl) |
Buffer H (μl) |
Final Volume (μl) |
| <partinfo>BBa_C0060</partinfo> |
Insert |
16.5 |
4 |
1 EcoRI |
1 SpeI |
2.5 |
25 |
| <partinfo>BBa_C0061</partinfo> |
Insert |
13.2 |
7.3 |
1 XbaI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_K081022</partinfo> |
Insert |
15.7 |
4.8 |
1 EcoRI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_B0030</partinfo> |
Vector |
13.2 |
7.3 |
1 SpeI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_B0031</partinfo> |
Vector |
12.4 |
8.1 |
1 SpeI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_B0032</partinfo> |
Vector |
9.5 |
11 |
1 SpeI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_B0015</partinfo> |
Vector |
7.9 |
12.6 |
1 EcoRI |
1 XbaI |
2.5 |
25 |
| <partinfo>pSB4C5</partinfo> |
Vector |
3 |
17.5 |
1 EcoRI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_I13501</partinfo> |
Insert |
7.2 |
13.3 |
1 XbaI |
1 PstI |
2.5 |
25 |
Reactions were incubated at 37°C for three hours while a small-size and a medium-size agarose gel were prepared according to protocols.
In the afternoon gel electrophoresis was performed.
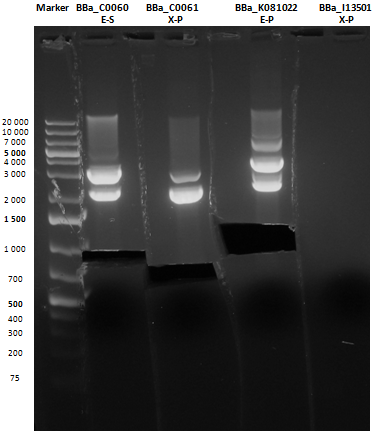
Small size gel
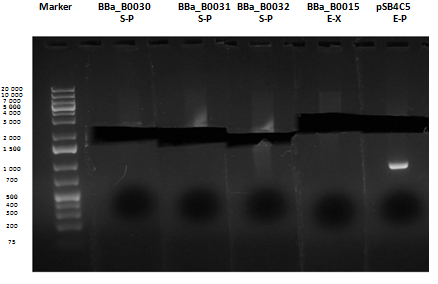
Medium size gel
As shown, in figure all clones were positive (apart from <partinfo>BBa_I13501</partinfo> run where we inexplicably didn't see any band), so we cut and purified the bands of interest. Cells harbouring <partinfo>BBa_I13501</partinfo> were again inoculated in 5 ml LB + Amp.
After gel extraction, cut DNA was quantified:
| Plasmid |
DNA (ng/μl) |
| <partinfo>BBa_C0060</partinfo> (E-S) |
3.9 |
| <partinfo>BBa_C0061</partinfo> (X-P) |
4.1 |
| <partinfo>BBa_K081022</partinfo> (E-P) |
4.4 |
| <partinfo>BBa_B0030</partinfo> (S-P) |
10.3 |
| <partinfo>BBa_B0031</partinfo> (S-P) |
11.8 |
| <partinfo>BBa_B0032</partinfo> (S-P) |
10.1 |
| <partinfo>BBa_B0015</partinfo> (E-X) |
12 |
| <partinfo>pSB4C5</partinfo> (E-P) |
10.7 |
Then ligations were performed in a final volume of 10 μl:
| Ligation Name |
Vector |
Vector volume (μl) |
Insert |
Insert volume (μl) |
Buffer (μl) |
T4 Ligase (μl) |
| E1 |
<partinfo>BBa_B0015</partinfo> (E-X) |
1.5 |
<partinfo>BBa_C0060</partinfo> (E-S) |
6.5 |
1 |
1 |
| E2 |
<partinfo>BBa_B0030</partinfo> (S-P) |
1.5 |
<partinfo>BBa_C0061</partinfo> (X-P) |
6.5 |
1 |
1 |
| E3 |
<partinfo>BBa_B0031</partinfo> (S-P) |
1.5 |
<partinfo>BBa_C0061</partinfo> (X-P) |
6.5 |
1 |
1 |
| E4 |
<partinfo>BBa_B0032</partinfo> (S-P) |
1.5 |
<partinfo>BBa_C0061</partinfo> (X-P) |
6.5 |
1 |
1 |
| E8 |
<partinfo>pSB4C5</partinfo> (E-P) |
1.5 |
<partinfo>BBa_K081022</partinfo> (E-P) |
6.5 |
1 |
1 |
Ligations were incubated ON at 16°C.
July, 5th
T4 Ligase was inactivated, heating ligations at 65°C for 10 minutes; ligations were stored at -20°C. Plasmid purification was done again for <partinfo>BBa_I13501</partinfo> and purified DNA was quantified:
| Plasmid |
DNA (ng/μl) |
| <partinfo>BBa_I13501</partinfo> |
97.2 |
<partinfo>BBa_I13501</partinfo> was then digested with restriction endonucleases:
| Plasmid |
Kind |
DNA (μl) |
H2O (μl) |
Enzyme 1 (μl) |
Enzyme 2 (μl) |
Buffer H (μl) |
Final Volume (μl) |
| <partinfo>BBa_I13501</partinfo> |
Insert |
10.5 |
10 |
1 XbaI |
1 PstI |
2.5 |
25 |
According to protocols the reaction was incubated at 37°C for 3 hours.
60 ml of 80% glycerol were prepared mixing 48 ml of 100% glycerol with 12 ml of ddH2O.
Gel electrophoresis was done for <partinfo>BBa_I13501</partinfo> digestion:

Small size gel
Cut DNA was gel-extracted:
| Plasmid |
DNA (ng/μl) |
| <partinfo>BBa_I13501</partinfo> (X-P) |
2.6 |
Further ligations were prepared and incubated ON at 16°C:
| Ligation Name |
Vector |
Vector volume (μl) |
Insert |
Insert volume (μl) |
Buffer (μl) |
T4 Ligase (μl) |
| E5 |
<partinfo>BBa_B0030</partinfo> (S-P) |
1 |
<partinfo>BBa_I13501</partinfo> (X-P) |
7 |
1 |
1 |
| E6 |
<partinfo>BBa_B0031</partinfo> (S-P) |
1 |
<partinfo>BBa_I13501</partinfo> (X-P) |
7 |
1 |
1 |
| E7 |
<partinfo>BBa_B0032</partinfo> (S-P) |
1 |
<partinfo>BBa_I13501</partinfo> (X-P) |
7 |
1 |
1 |
 "
"


