Team:NYMU-Taipei/results/immunological-solution1
From 2011.igem.org
(→(i) Magnetotactic bacteria were introduced into guanulocytes and monocytes by phagocytosis.(Tadashi Matsunaga et all. 1986)) |
(→Measurements of Construct tetR+rbs+gfp under Fluorescence Microscope) |
||
| (19 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
==<font size=5><font color=crimson>'''Constructs & Parts '''</font></font>== | ==<font size=5><font color=crimson>'''Constructs & Parts '''</font></font>== | ||
| - | ===<font size=4><font color=green>'''( | + | ===<font size=4><font color=green>'''(1) <i>MinC</i> Construct and <i>Inv</i> & <i>LLO</i> Constructs on AMB-1'''</font></font>=== |
<center> | <center> | ||
{| class="wikitable" border="1" | {| class="wikitable" border="1" | ||
| Line 10: | Line 10: | ||
! [[Image: AMB-1_Inv_LLO.jpg|center]] | ! [[Image: AMB-1_Inv_LLO.jpg|center]] | ||
|- style="text-align:center;" | |- style="text-align:center;" | ||
| - | | Fig. Design of <i>minC</i> construct on AMB-1<br>Backbone plasmid: pYMB | + | | <font size=3>Fig. Design of <i>minC</i> construct on AMB-1<br>Backbone plasmid: pYMB</font> |
| - | | Fig. Design of <i>Inv</i> & <i>LLO</i> construct on AMB-1<br>Backbone plasmid: pRKm415 | + | | <font size=3>Fig. Design of <i>Inv</i> & <i>LLO</i> construct on AMB-1<br>Backbone plasmid: pRKm415</font> |
|} | |} | ||
</center> | </center> | ||
| - | ===<font size=4><font color=green>'''( | + | ===<font size=4><font color=green>'''(2) Related Parts'''</font></font>=== |
<font size=3>We have created several parts on the basis of Biobrick. The expression will be shown on the partregistry webpage. | <font size=3>We have created several parts on the basis of Biobrick. The expression will be shown on the partregistry webpage. | ||
| Line 52: | Line 52: | ||
</font> | </font> | ||
| - | ===<font size=4><font color=green>'''( | + | ===<font size=4><font color=green>'''(3) Nested-PCR Primers for Various Strains'''</font></font>=== |
| - | <font size=4><font color=blue>'''nested-Inv'''</font></font> | + | <font size=4><font color=blue>'''(i) nested-Inv'''</font></font> |
<font size=3> | <font size=3> | ||
{| border=1 | {| border=1 | ||
| Line 64: | Line 64: | ||
</font> | </font> | ||
| - | <font size=4><font color=blue>'''nested-LLO'''</font></font> | + | <font size=4><font color=blue>'''(ii) nested-LLO'''</font></font> |
<font size=3> | <font size=3> | ||
{| border=1 | {| border=1 | ||
| Line 75: | Line 75: | ||
</font> | </font> | ||
| - | ===<font size=4><font color=green>'''( | + | ===<font size=4><font color=green>'''(4) Results of PCR from genomic DNA'''</font></font>=== |
<center> | <center> | ||
{| class="wikitable" border="0" | {| class="wikitable" border="0" | ||
| Line 83: | Line 83: | ||
! [[Image:gel_minC.png]] | ! [[Image:gel_minC.png]] | ||
|- style="text-align:center;" | |- style="text-align:center;" | ||
| - | | Fig. <i>invasin</i> PCR cloned from<br><i>Yersinia enterocolitica</i> | + | | <font size=3>Fig. <i>invasin</i> PCR cloned from<br><i>Yersinia enterocolitica</i></font> |
| - | | Fig. <i>LLO</i> PCR cloned from<br><i>Listeria monocytogenes</i> | + | | <font size=3>Fig. <i>LLO</i> PCR cloned from<br><i>Listeria monocytogenes</i></font> |
| - | | Fig. <i>minC</i> PCR cloned from<br><i>E.coli</i> K12 MG1655 | + | | <font size=3>Fig. <i>minC</i> PCR cloned from<br><i>E.coli</i> K12 MG1655</font> |
|} | |} | ||
</center> | </center> | ||
| - | ===<font size=4><font color=green>'''( | + | ===<font size=4><font color=green>'''(5) Expression of TetR Protein Enabled Regulation'''</font></font>=== |
<center> | <center> | ||
{| class="wikitable" border="0" | {| class="wikitable" border="0" | ||
| Line 95: | Line 95: | ||
! [[Image:expression_of_tetR_protein.png]] | ! [[Image:expression_of_tetR_protein.png]] | ||
|- | |- | ||
| - | | Fig. From the left to right, there are separately <b>marker</b> labelled at 72kDa and 26kDa, <br><b>supernatant control</b>, <b>supernatant experiment</b>, <b>pellet control</b>, <b>pellet experiment</b>. | + | | <font size=3>Fig. From the left to right, there are separately <b>marker</b> labelled at 72kDa and 26kDa, <br><b>supernatant control</b>, <b>supernatant experiment</b>, <b>pellet control</b>, <b>pellet experiment</b>.</font> |
|} | |} | ||
</center> | </center> | ||
<font size=3>Tetracyclin resistent protein and GFP were induced by IPTG to express in BL-21 <i>E.coli</i> strain for the confirmation of the construct.As SDS-PAGE shown above, there is no difference between supernatant control and experiment while tetracyclin resistent protein and GFP express in the pellet experiment.It is proved that the expression of tetracyclin resistent protein can enable the regulation.</font> | <font size=3>Tetracyclin resistent protein and GFP were induced by IPTG to express in BL-21 <i>E.coli</i> strain for the confirmation of the construct.As SDS-PAGE shown above, there is no difference between supernatant control and experiment while tetracyclin resistent protein and GFP express in the pellet experiment.It is proved that the expression of tetracyclin resistent protein can enable the regulation.</font> | ||
| - | ==<font size=5><font color=crimson>'''Symbiosis within Human Glial | + | ==<font size=5><font color=crimson>'''Symbiosis within Human Glial Cells '''</font></font>== |
| - | ===<font size=4><font color=green>'''<b>( | + | ===<font size=4><font color=green>'''<b>(1)</b> <font size=3>Magnetotactic bacteria were introduced into guanulocytes and monocytes by phagocytosis.(Tadashi Matsunaga et all. 1986)</font></font>=== |
<center> | <center> | ||
{| class="wikitable" border="0" | {| class="wikitable" border="0" | ||
|- | |- | ||
| - | + | ! [[Image:monocyte.jpg]] | |
| - | |- | + | |- style="text-align:center;" |
| - | | Fig.The transmission electron micrograph(TEM) of a monocyte that had ingested magnetotactic bacteria. The bar represents 5 μm. | + | | <font size=3>Fig.The transmission electron micrograph(TEM) of a monocyte <br>that had ingested magnetotactic bacteria. The bar represents 5 μm.</font> |
|} | |} | ||
</center> | </center> | ||
| - | |||
| - | ===<font size=4><font color=green>'''<b>( | + | ===<font size=4><font color=green>'''<b>(2)</b> In our experiment, AMB-1 was introduced into primary mixed glial cells <i>in vitro</i>. Figures 1~4 show the photos captured under the microscope.</font></font>=== |
'''<font size=3>Check out [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624032 BBa_K624032] for detailed characterization</font>''' | '''<font size=3>Check out [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624032 BBa_K624032] for detailed characterization</font>''' | ||
| - | <center | + | <center> |
{| class="wikitable" border="1" | {| class="wikitable" border="1" | ||
| - | |- | + | |- style="text-align:center;" |
| - | | [[Image: AMB-1_glial1.jpg|center|400px|thumb|Fig.1 primary mixed glial cell | + | | [[Image: AMB-1_glial1.jpg|center|400px|thumb|<font size=3>Fig.1 primary mixed glial cell introduced with AMB-1 1</font>]] |
| - | | [[Image: AMB-1_glial2.jpg|center|400px|thumb|Fig.2 primary mixed glial cell | + | | [[Image: AMB-1_glial2.jpg|center|400px|thumb|<font size=3>Fig.2 primary mixed glial cell introduced with AMB-1 2</font>]] |
| - | |- | + | |- style="text-align:center;" |
| - | | [[Image: glial1.jpg|center|400px|thumb|Fig.3 primary mixed glial cell control 1]] | + | | [[Image: glial1.jpg|center|400px|thumb|<font size=3>Fig.3 primary mixed glial cell control 1</font>]] |
| - | | [[Image: glial2.jpg|center|400px|thumb|Fig.4 primary mixed glial cell control 2]] | + | | [[Image: glial2.jpg|center|400px|thumb|<font size=3>Fig.4 primary mixed glial cell control 2</font>]] |
|} | |} | ||
| - | + | </center> | |
==<font size=5><font color=crimson>'''Measurements of Construct <i>tetR</i>+<i>rbs</i>+<i>gfp</i> under Fluorescence Microscope'''</font></font>== | ==<font size=5><font color=crimson>'''Measurements of Construct <i>tetR</i>+<i>rbs</i>+<i>gfp</i> under Fluorescence Microscope'''</font></font>== | ||
| Line 136: | Line 135: | ||
|} | |} | ||
</center> | </center> | ||
| - | <font size=3>These are the measurements of our construct [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624001 '''<u><i>tetR</i>+<i>rbs</i>+<i>GFP</i></u>]'''(BBa_K624001) under fluorescence microscope. '''The left | + | <font size=3>These are the measurements of our construct [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624001 '''<u><i>tetR</i>+<i>rbs</i>+<i>GFP</i></u>]'''(BBa_K624001) under fluorescence microscope. '''The picture on the left''' is from the control group of ''<i>E. coli</i>'' strain DH5-alpha. '''The middle''' is <i>tetR</i>+<i>rbs</i>+<i>gfp</i> in plasmid pSB1C3 in ''<i>E. coli</i>'', while '''the right''' is from a plate without any bacilli. All graphs are measured in fixed exposure time. Construct of <i>tetR</i>+<i>rbs</i>+<i>gfp</i> which emits light is thought to be a leak phenomenon of plasmid, while RE digest has proved that the sequence length is correct, and further sequencing work is under operation. |
<font size=4><font color=green>'''<i>gfp</i> measurements under different excitation wavelengths'''</font></font> | <font size=4><font color=green>'''<i>gfp</i> measurements under different excitation wavelengths'''</font></font> | ||
Latest revision as of 21:51, 28 October 2011

Contents |
Constructs & Parts
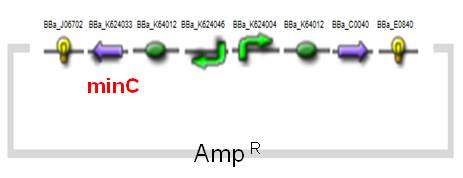
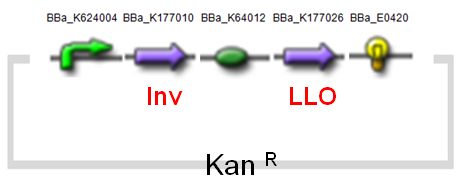
(1) MinC Construct and Inv & LLO Constructs on AMB-1
| Fig. Design of minC construct on AMB-1 Backbone plasmid: pYMB | Fig. Design of Inv & LLO construct on AMB-1 Backbone plasmid: pRKm415 |
(2) Related Parts
We have created several parts on the basis of Biobrick. The expression will be shown on the partregistry webpage.
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624033 BBa_K624033] | minC cell division inhibitor (revised) |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624034 BBa_K624034] | revised minC primer (forward) |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624035 BBa_K624035] | revised minC primer (reverse) |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624036 BBa_K624036] | minC+mcherry |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624037 BBa_K624037] | rbs+minC+mcherry |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624040 BBa_K624040] | pT7-tetO+rbs+minC |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624045 BBa_K624045] | Pmsp1(tetO) + rbs(Pmsp3) + minC + rbs(Pmsp3) + mcherry |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624056 BBa_K624056] | pYMB essentials + rbs(Pmsp3) + minC |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624057 BBa_K624057] | pYMB essentials + rbs(Pmsp3) + LLO |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624058 BBa_K624058] | pYMB essentials + rbs(Pmsp3) + Inv |
(3) Nested-PCR Primers for Various Strains
(i) nested-Inv
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624063 BBa_K624063] | nested_Inv F (inter-strain nested primer) |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624064 BBa_K624064] | nested_Inv R (inter-strain nested primer) |
(ii) nested-LLO
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624065 BBa_K624065] | nested_LLO F (inter-strain nested primer) |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624066 BBa_K624066] | nested_LLO R (inter-strain nested primer) |
(4) Results of PCR from genomic DNA

| 
| 
|
|---|---|---|
| Fig. invasin PCR cloned from Yersinia enterocolitica | Fig. LLO PCR cloned from Listeria monocytogenes | Fig. minC PCR cloned from E.coli K12 MG1655 |
(5) Expression of TetR Protein Enabled Regulation

|
|---|
| Fig. From the left to right, there are separately marker labelled at 72kDa and 26kDa, supernatant control, supernatant experiment, pellet control, pellet experiment. |
Tetracyclin resistent protein and GFP were induced by IPTG to express in BL-21 E.coli strain for the confirmation of the construct.As SDS-PAGE shown above, there is no difference between supernatant control and experiment while tetracyclin resistent protein and GFP express in the pellet experiment.It is proved that the expression of tetracyclin resistent protein can enable the regulation.
Symbiosis within Human Glial Cells
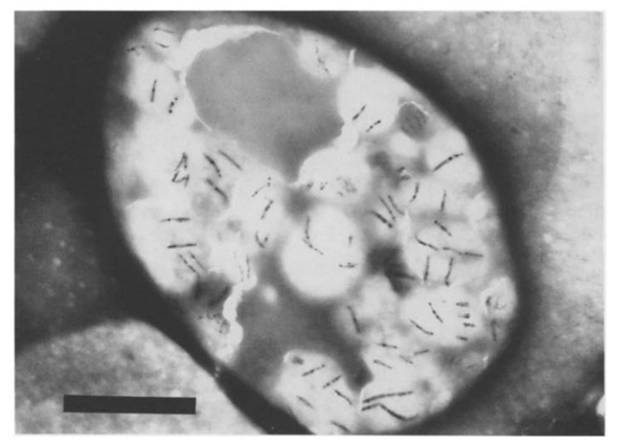
(1) Magnetotactic bacteria were introduced into guanulocytes and monocytes by phagocytosis.(Tadashi Matsunaga et all. 1986)
|
|---|
| Fig.The transmission electron micrograph(TEM) of a monocyte that had ingested magnetotactic bacteria. The bar represents 5 μm. |
(2) In our experiment, AMB-1 was introduced into primary mixed glial cells in vitro. Figures 1~4 show the photos captured under the microscope.
Check out [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624032 BBa_K624032] for detailed characterization
Measurements of Construct tetR+rbs+gfp under Fluorescence Microscope
| 
| 
|
|---|
These are the measurements of our construct [http://partsregistry.org/wiki/index.php?title=Part:BBa_K624001 tetR+rbs+GFP](BBa_K624001) under fluorescence microscope. The picture on the left is from the control group of E. coli strain DH5-alpha. The middle is tetR+rbs+gfp in plasmid pSB1C3 in E. coli, while the right is from a plate without any bacilli. All graphs are measured in fixed exposure time. Construct of tetR+rbs+gfp which emits light is thought to be a leak phenomenon of plasmid, while RE digest has proved that the sequence length is correct, and further sequencing work is under operation.
gfp measurements under different excitation wavelengths
The expression of gfp is measured precisely under excitation wavelength from 475nm to 501nm, given the absorption wavelength 511nm. In our measurement, wavelength over 493nm yields high background, resulting in values of control group surging far beyond normal condition. Thus, 493nm is suggested as the best excitation wavelength, as it presents the highest emission light and the lowest of the control group.
 "
"