Team:Uppsala-Sweden/Characterization
From 2011.igem.org
Tomasdalmo (Talk | contribs) |
|||
| (10 intermediate revisions not shown) | |||
| Line 18: | Line 18: | ||
| - | <div class=" | + | <div class="grid_Orange_thin omega mywhite"> |
Test for light-sensor and promoter characterization | Test for light-sensor and promoter characterization | ||
</div> | </div> | ||
Characterization is an important aspect of synthetic biology and it is a way of obtaining how well the expression of the system really is. Therefore we have designed two tests to perform characterization of the parts we are going to use. Both types of test are based on the expression levels of BFP. The first test is done in order to characterize the promoters that we are using in our project. The second test is done to test the output of our light sensing system. The expression levels will in both cases be measured using flow-cytometry. Here the specific details of how the test has/will been performed depending on its type. | Characterization is an important aspect of synthetic biology and it is a way of obtaining how well the expression of the system really is. Therefore we have designed two tests to perform characterization of the parts we are going to use. Both types of test are based on the expression levels of BFP. The first test is done in order to characterize the promoters that we are using in our project. The second test is done to test the output of our light sensing system. The expression levels will in both cases be measured using flow-cytometry. Here the specific details of how the test has/will been performed depending on its type. | ||
| - | <div class=" | + | |
| + | <br><br> | ||
| + | <div class="grid_Red_thin omega mywhite"> | ||
Promoter testing | Promoter testing | ||
</div> | </div> | ||
<br> | <br> | ||
| - | The first test was done to see the strength of the promoters used. From the beginning it was meant to be a characterization of the promoter Pt5lac that we provide to the partsregistry. Though since the other promoters we are using which were gained from the partsregistry was not characterized yet, the decision was made that we would have to characterize all the promoters we use. Since, as said in our opinion characterization is a really important aspect of the working approach in synthetic biology. | + | The first test was done to see the strength of the promoters used. From the beginning it was meant to be a characterization of the promoter Pt5lac that we provide to the partsregistry. Though since the other promoters we are using which were gained from the partsregistry was not characterized yet and as we used some other new promoters not yet in the registry, the decision was made that we would have to characterize all the promoters we use. Since, as said in our opinion characterization is a really important aspect of the working approach in synthetic biology. |
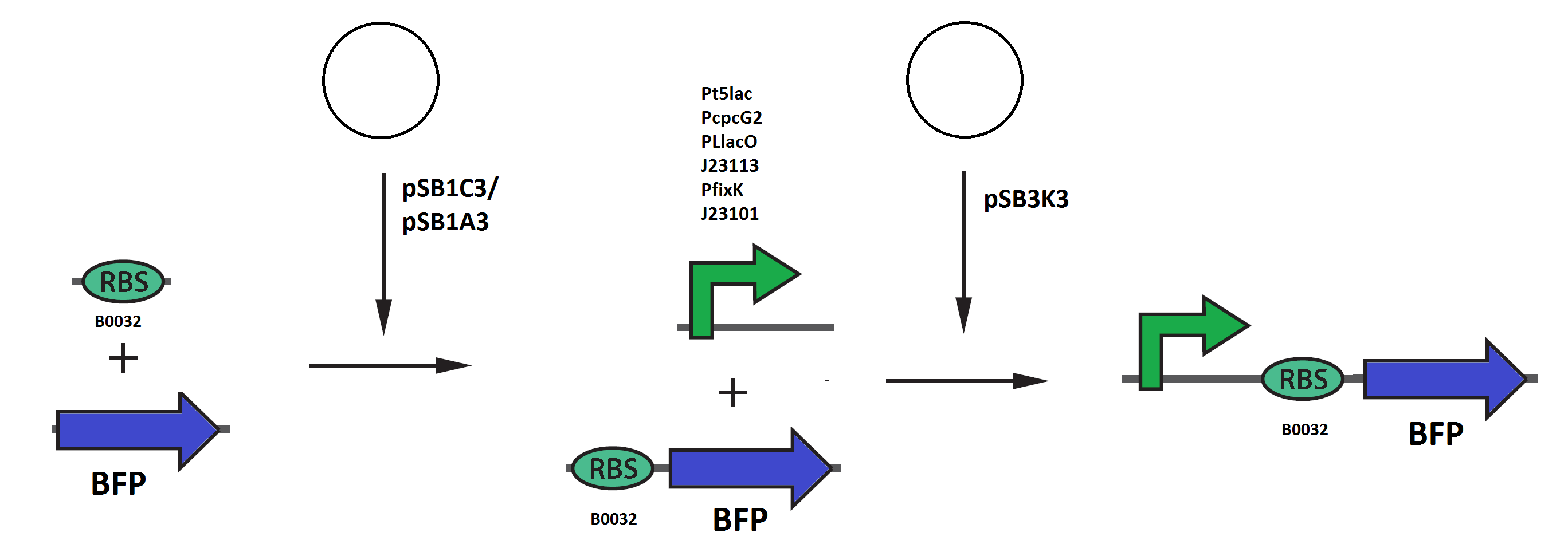
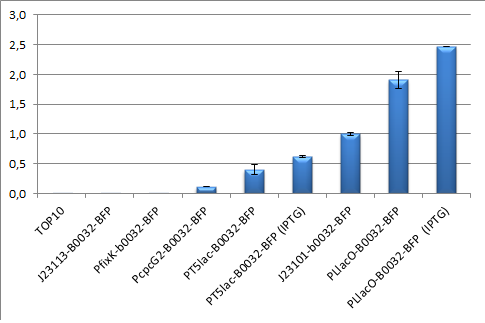
| - | Every construct was made according to the approach provided by the measurement page in partsregistry besides from that the expressed protein was BFP. This means that the backbone pSB3K3, the RBS B0032 and | + | Every construct was made according to the approach provided by the measurement page in partsregistry besides from that the expressed protein was BFP. This means that the backbone pSB3K3, the RBS B0032 and TagBFP were used in every construct. The promoters tested were pT5lac, PcpcG2, PLlacO, J23113, PfixK and as reference promoter J23101. The assemblies were done according to the picture below and the result from the promoter test is showed below to the right. |
| + | <br> | ||
| + | [[Image:Promoter_assebmly.png|320 px|left]] | ||
| + | [[Image:Promoter_characterization.png|320 px|left]] | ||
| + | <br><br><br><br><br><br><br><br><br><br><br> | ||
| - | The actual test was done in a | + | The actual test was done in a slightly different way than that presented at the partsregistry. Here we use flow-cytometry which also results in that we did not use M9 media to grow the cells but used LB instead. Also when it comes to the time of incubation of the selected colonies which were 10 hours and no dilutions were made except for the settings requirements of the FACS machine. |
| - | + | ||
| - | + | ||
| + | <br><br> | ||
<div class="grid_Blue_thin omega mywhite"> | <div class="grid_Blue_thin omega mywhite"> | ||
System output tests | System output tests | ||
</div> | </div> | ||
| - | This test will be fully compatible with our system. The only change is that every constructed output will be done with | + | This test will be fully compatible with our system. The only change is that every constructed output will be done with TagBFP as output instead of the color output in order to get a preference of how efficient the light-sensors are. Since there are no fully functional sensor yet, no tests has been able to be performed. Though Both the corresponding blue and yellow output is ready to be used. The pictures presented below shows the asseblies of each output corresponding to its sensor module, but as said here with TagBFP as output. |
| + | <br> | ||
| + | '''TagBFP output modules''' | ||
| + | <br> | ||
| + | [[Image:Blueoutputassembly.png|600px|thumb|left|Blue Output]]<br> | ||
| + | [[Image:Redoutputassembly.png|600px|thumb|left|Red Output]]<br> | ||
| + | [[Image:Yellowoutputassembly.png|600px|thumb|left|Yellow Output]]<br> | ||
{{Template:Uppsala-SwedenTemplatefooter}} | {{Template:Uppsala-SwedenTemplatefooter}} | ||
Latest revision as of 13:56, 25 October 2011
Test for light-sensor and promoter characterization
Characterization is an important aspect of synthetic biology and it is a way of obtaining how well the expression of the system really is. Therefore we have designed two tests to perform characterization of the parts we are going to use. Both types of test are based on the expression levels of BFP. The first test is done in order to characterize the promoters that we are using in our project. The second test is done to test the output of our light sensing system. The expression levels will in both cases be measured using flow-cytometry. Here the specific details of how the test has/will been performed depending on its type.
Promoter testing
The first test was done to see the strength of the promoters used. From the beginning it was meant to be a characterization of the promoter Pt5lac that we provide to the partsregistry. Though since the other promoters we are using which were gained from the partsregistry was not characterized yet and as we used some other new promoters not yet in the registry, the decision was made that we would have to characterize all the promoters we use. Since, as said in our opinion characterization is a really important aspect of the working approach in synthetic biology.
Every construct was made according to the approach provided by the measurement page in partsregistry besides from that the expressed protein was BFP. This means that the backbone pSB3K3, the RBS B0032 and TagBFP were used in every construct. The promoters tested were pT5lac, PcpcG2, PLlacO, J23113, PfixK and as reference promoter J23101. The assemblies were done according to the picture below and the result from the promoter test is showed below to the right.
The actual test was done in a slightly different way than that presented at the partsregistry. Here we use flow-cytometry which also results in that we did not use M9 media to grow the cells but used LB instead. Also when it comes to the time of incubation of the selected colonies which were 10 hours and no dilutions were made except for the settings requirements of the FACS machine.
System output tests
This test will be fully compatible with our system. The only change is that every constructed output will be done with TagBFP as output instead of the color output in order to get a preference of how efficient the light-sensors are. Since there are no fully functional sensor yet, no tests has been able to be performed. Though Both the corresponding blue and yellow output is ready to be used. The pictures presented below shows the asseblies of each output corresponding to its sensor module, but as said here with TagBFP as output.
TagBFP output modules
 "
"