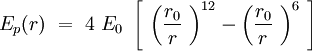Team:Paris Bettencourt/Modeling/Assisted diffusion/Membrane tension
From 2011.igem.org
(Created page with "bla bla") |
|||
| Line 1: | Line 1: | ||
| - | + | === When membranes behave like 2D Van der Waals fluids === | |
| + | |||
| + | Trapped between the osmotic pressure and the cell wall, phospholipids are moving in a double layered environment. Their motion is constricted into a two dimensional motion, following the shape of the membrane. At the scale of the phospholipid, the motion can be approximated to a motion in 2D. Using statistical physics approach, we can say that the phase space has 4N dimensions: two dimensions of impulsion and two dimensions of coordinate for each particle (N is the number of particles in the system). | ||
| + | |||
| + | Can we describe the interaction between two phospholipids in the membrane? The question is complicated, but we will demonstrate that by describing properly the phospholipid, as it is reasonable to fit a Lennard-Jones potential energy to this interaction. The energy of binding is smaller and the speed of sliding of one phospholipid against another is slower than the internal vibration of the chemical bounds and the internal conformers rotation of the CH2 tails. Though, in ''Intermolecular Forces'' by Israelachvili, the author shows that a phospholipid trapped in a bilayer can be aproximated by a section of cone. The section of cones is an individual, and the others are sections of cone joining the previous one. The shape of the sections are giving the shape of the global structure. | ||
| + | |||
| + | <html> | ||
| + | <br/> | ||
| + | <table> | ||
| + | <tr> | ||
| + | <td align="center"><img src="https://static.igem.org/mediawiki/2011/e/e9/Image_phospholipide.png" height=200px></td> | ||
| + | <td align="center"><img src="https://static.igem.org/mediawiki/2011/8/82/Mod%C3%A8le_Phospholipide.png" height=200px></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td align="center"><center><b><u>Fig5:</u></b> Chemical structure of one phospholipid</center></td> | ||
| + | <td align="center"><center><b><u>Fig6:</u></b> Schematics of the cylindrical approximation made for the phospholipid</center></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br/> | ||
| + | </html> | ||
| + | |||
| + | Speaking in terms of energy, once we have these cones, it is reasonable to fit a Lennard-Jones potential to this interaction. If the cones are interpenetrating, a steric repulsion keeps the molecules apart, and the hydrophobic interaction that joins one lipid with the other acts as an attractive force keeping the coherence of the membrane. We will discuss later about the value of the two coefficients. | ||
| + | |||
| + | [[File:LJpot.jpg|center|Lennard-Jones potential]] | ||
| + | |||
| + | == The membrane tension calculation == | ||
| + | <html> | ||
| + | |||
| + | Considering one membrane a sphere of the radius R, on which the phospholipid double layer is uniformally distributed, we can find approximately a part of the whole sphere surface per one phospholipid : | ||
| + | |||
| + | <p> | ||
| + | <center> | ||
| + | <img src='https://static.igem.org/mediawiki/2011/f/fa/Surface_per_phospholipid.png'> | ||
| + | </center> | ||
| + | </p> | ||
| + | |||
| + | The caracteristic distance between two neighbor phospholipids on the membrane can be written like this : | ||
| + | |||
| + | <p> | ||
| + | <center> | ||
| + | <img src='https://static.igem.org/mediawiki/2011/c/c6/Characteristical_length_between_2_phl.png'> | ||
| + | </center> | ||
| + | </p> | ||
| + | |||
| + | where N is the number of phospholipids on the membrane. | ||
| + | |||
| + | From this moment on we will work in canonical ensemble. The temperature is fixed and so is the number of particles in the system N. We also consider that the phospholipids can move only on the sphere surface that means that we will study the evolution of our system in 2N dimentional phase space. | ||
| + | |||
| + | The partition function will look like : | ||
| + | |||
| + | <p> | ||
| + | <center> | ||
| + | <img src='https://static.igem.org/mediawiki/2011/2/27/Partition_function.png'> | ||
| + | </center> | ||
| + | </p> | ||
| + | |||
| + | where the Hamiltonian is : | ||
| + | |||
| + | <p> | ||
| + | <center> | ||
| + | <img src='https://static.igem.org/mediawiki/2011/b/be/Hamiltonian.png' style='width:100%;'> | ||
| + | </center> | ||
| + | </p> | ||
| + | |||
| + | Knowing the partition function is a great deal. We can derive a free energy | ||
| + | |||
| + | <p> | ||
| + | <center> | ||
| + | <img src='https://static.igem.org/mediawiki/2011/c/cf/Free_energy.png'> | ||
| + | </center> | ||
| + | </p> | ||
| + | |||
| + | of each sphere, total free energy and the membranian tenstion from it : | ||
| + | |||
| + | <p> | ||
| + | <center> | ||
| + | <img src='https://static.igem.org/mediawiki/2011/4/49/Free_energy_total.png' style='width:100%;'> | ||
| + | </center> | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | <center> | ||
| + | <img src='https://static.igem.org/mediawiki/2011/f/f4/Tension.png'> | ||
| + | </center> | ||
| + | </p> | ||
| + | |||
| + | For two spheres we get : | ||
| + | |||
| + | <p> | ||
| + | <center> | ||
| + | <img src='https://static.igem.org/mediawiki/2011/f/f2/Tension_1.png'> | ||
| + | </center> | ||
| + | </p> | ||
| + | |||
| + | and | ||
| + | |||
| + | <p> | ||
| + | <center> | ||
| + | <img src='https://static.igem.org/mediawiki/2011/8/87/Tension_2.png'> | ||
| + | </center> | ||
| + | </p> | ||
| + | |||
| + | </html> | ||
Revision as of 14:50, 23 October 2011
When membranes behave like 2D Van der Waals fluids
Trapped between the osmotic pressure and the cell wall, phospholipids are moving in a double layered environment. Their motion is constricted into a two dimensional motion, following the shape of the membrane. At the scale of the phospholipid, the motion can be approximated to a motion in 2D. Using statistical physics approach, we can say that the phase space has 4N dimensions: two dimensions of impulsion and two dimensions of coordinate for each particle (N is the number of particles in the system).
Can we describe the interaction between two phospholipids in the membrane? The question is complicated, but we will demonstrate that by describing properly the phospholipid, as it is reasonable to fit a Lennard-Jones potential energy to this interaction. The energy of binding is smaller and the speed of sliding of one phospholipid against another is slower than the internal vibration of the chemical bounds and the internal conformers rotation of the CH2 tails. Though, in Intermolecular Forces by Israelachvili, the author shows that a phospholipid trapped in a bilayer can be aproximated by a section of cone. The section of cones is an individual, and the others are sections of cone joining the previous one. The shape of the sections are giving the shape of the global structure.
 |
 |
Speaking in terms of energy, once we have these cones, it is reasonable to fit a Lennard-Jones potential to this interaction. If the cones are interpenetrating, a steric repulsion keeps the molecules apart, and the hydrophobic interaction that joins one lipid with the other acts as an attractive force keeping the coherence of the membrane. We will discuss later about the value of the two coefficients.
The membrane tension calculation
Considering one membrane a sphere of the radius R, on which the phospholipid double layer is uniformally distributed, we can find approximately a part of the whole sphere surface per one phospholipid :









 "
"