Team:TzuChiU Formosa/Notebook/photopaper
From 2011.igem.org
(Difference between revisions)
(→2011.09.10-11) |
|||
| (144 intermediate revisions not shown) | |||
| Line 25: | Line 25: | ||
</html> | </html> | ||
| + | <font color="#228B22" size=6>Photopaper</font> | ||
| + | <br><br><font color="#000080" size=4>Meeting Minutes</font> | ||
| + | <Hr Align="left" width="100%" size=2> | ||
| + | <font color="#000000" size=3><b>2011.02.24</b></font> | ||
| + | <br><font size=2><b>Discussion:</b></font> | ||
| - | |||
| - | |||
| - | |||
| - | |||
*Team organization[[File:001.jpg|right|500px|caption]] | *Team organization[[File:001.jpg|right|500px|caption]] | ||
*Brain storming | *Brain storming | ||
| Line 36: | Line 37: | ||
| - | == | + | |
| - | + | <br><font color="#000000" size=3><b>2011.03.04</b></font> | |
| + | <br><font size=2><b>Discussion:</b></font> | ||
| + | |||
*Team advisory | *Team advisory | ||
*Brain storming | *Brain storming | ||
| Line 44: | Line 47: | ||
| - | == | + | |
| - | + | ||
| + | <br><font color="#000000" size=3><b>2011.03.14</b></font> | ||
| + | <br><font size=2><b>Discussion:</b></font> | ||
| + | |||
*Task Allocation | *Task Allocation | ||
*Brain storming | *Brain storming | ||
| Line 52: | Line 58: | ||
| - | == | + | |
| - | + | <br><font color="#000000" size=3><b>2011.03.23</b></font> | |
| + | <br><font size=2><b>Discussion:</b></font> | ||
**Project : paperia | **Project : paperia | ||
| Line 60: | Line 67: | ||
| - | == | + | |
| - | + | <br><font color="#000000" size=3><b>2011.03.24</b></font> | |
| + | <br><font size=2><b>Discussion:</b></font> | ||
* Exp. procedure: | * Exp. procedure: | ||
| Line 68: | Line 76: | ||
| - | == | + | |
| - | + | <br><font color="#000000" size=3><b>2011.06.22</b></font> | |
| + | <br><font size=2><b>Discussion:</b></font> | ||
| + | |||
* Due to some unforseen reason, the team decided to change their project. | * Due to some unforseen reason, the team decided to change their project. | ||
* New project: Biojenny | * New project: Biojenny | ||
| Line 75: | Line 85: | ||
-yeast to be our host | -yeast to be our host | ||
| - | == | + | |
| - | + | ||
| + | <br><font color="#000000" size=3><b>2011.07.01</b></font> | ||
| + | <br><font size=2><b>Discussion:</b></font> | ||
* Freeze > grin > genome DNA isolation > Cloning = silk protein gene | * Freeze > grin > genome DNA isolation > Cloning = silk protein gene | ||
| - | == | + | |
| - | + | <br><font color="#000000" size=3><b>2011.07.09</b></font> | |
| + | <br><font size=2><b>Discussion:</b></font> | ||
| + | |||
* the connections between 3 silk proteins : Fibl Fibh P25 | * the connections between 3 silk proteins : Fibl Fibh P25 | ||
* major proteins : H-chain, L-chain, P25 | * major proteins : H-chain, L-chain, P25 | ||
| - | == | + | |
| - | + | <br><font color="#000000" size=3><b>2011.07.15</b></font> | |
| + | <br><font size=2><b>Discussion:</b></font> | ||
| + | |||
* Due to limited resources and techniques required, the team decided to switch back to the previous project. Paper making ! | * Due to limited resources and techniques required, the team decided to switch back to the previous project. Paper making ! | ||
* However it would be modified to be more innovative and creative. | * However it would be modified to be more innovative and creative. | ||
| - | == | + | |
| - | + | <br><font color="#000000" size=3><b>2011.07.18</b></font> | |
| + | <br><font size=2><b>Discussion:</b></font> | ||
| + | |||
* Latest project : Photo paper | * Latest project : Photo paper | ||
* cyanobacteria is designed as the host, cellulose made up of the glucose produced by cyanobacteria could be one of the main attraction of the project. | * cyanobacteria is designed as the host, cellulose made up of the glucose produced by cyanobacteria could be one of the main attraction of the project. | ||
| - | == | + | |
| - | + | <br><font color="#000000" size=3><b>2011.07.23</b></font> | |
| + | <br><font size=2><b>Discussion:</b></font> | ||
* system modification to overcome the problems arises during preliminary round | * system modification to overcome the problems arises during preliminary round | ||
| Line 107: | Line 126: | ||
| - | == | + | |
| + | |||
| + | <br><font color="#000000" size=3><b>2011.09.15</b></font> | ||
'''Genome miniprep'''<br> | '''Genome miniprep'''<br> | ||
| Line 113: | Line 134: | ||
| - | == | + | |
| + | <br><font color="#000000" size=3><b>2011.09.18</b></font> | ||
'''Gel/PCR DNA extraction'''<br> | '''Gel/PCR DNA extraction'''<br> | ||
Gluconacetobacter hansenii | Gluconacetobacter hansenii | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | <font size=4 color="#000080"><b>Protocols</b></font> | |
| + | <Hr Align="left" width="100%" size=3> | ||
| - | |||
| - | |||
| - | < | + | <br>[[File:1-20110907.jpg|lefe|650px|caption]][[File:Catt.gif|right|250px|caption]] |
| - | File: | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | 1 | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | File: | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<br> | <br> | ||
| - | |||
<br> | <br> | ||
| + | <br>[[File:2-20110908-13.jpg|lefe|650px|caption]] | ||
<br> | <br> | ||
<br> | <br> | ||
| - | |||
<br> | <br> | ||
| - | + | <br>[[File:3-20110910-11.jpg|lefe|650px|caption]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<br> | <br> | ||
| + | <br>[[File:4-20110912-20.jpg|lefe|650px|caption]] | ||
<br> | <br> | ||
<br> | <br> | ||
| - | |||
<br> | <br> | ||
| - | [ | + | <br>[[File:5-20110921.jpg|lefe|550px|caption]][[File:Cats.jpg|right|404px|caption]] |
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br>[[File:6-20110922.jpg|lefe|650px|caption]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br>[[File:7-20110923.jpg|lefe|650px|caption]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br>[[File:8-20110924.jpg|lefe|550px|caption]][[File:Cats2.gif |right|404px|caption]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br>[[File:9-20110925.jpg|lefe|650px|caption]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br>[[File:10-20110926.jpg|lefe|650px|caption]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br>[[File:11-20110927.jpg|lefe|550px|caption]][[File:Cats3.jpg|right|404px|caption]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br>[[File:12-20110928.jpg|lefe|650px|caption]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br>[[File:13-20110929.jpg|lefe|650px|caption]] | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br>[[File:14-20111001.jpg|lefe|650px|caption]] | ||
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Latest revision as of 02:56, 6 October 2011


Photopaper
Meeting Minutes
2011.02.24
Discussion:
- Team organization
- Brain storming
- paper made by bacteria with add-ons such as colors, fragrance, etc.
- "light up" the plants for replacing lamp posts.
2011.03.04
Discussion:
- Team advisory
- Brain storming
- Add-ons: colorful cellulose which produced by bacteria such as beta-carotene
- information exchange with iGEM 2009 Cambridge team
2011.03.14
Discussion:
- Task Allocation
- Brain storming
- Avatar - "light up" the plants by transfecting symbiotic bacteria which cloned into fluorescence gene
- Eco-friendly warmer - biotic thermal pad
2011.03.23
Discussion:
- Project : paperia
- Option 1 : Culture bacteria which has pigment gene
- Option 2 : Cellulose-producing bacteria secrete pigment into the medium
2011.03.24
Discussion:
- Exp. procedure:
- cloning of cellulose gene’s CDS
- the product should operate within E. coli.
2011.06.22
Discussion:
- Due to some unforseen reason, the team decided to change their project.
- New project: Biojenny
-economical and humane way to produce paper in large quantities.
-yeast to be our host
2011.07.01
Discussion:
- Freeze > grin > genome DNA isolation > Cloning = silk protein gene
2011.07.09
Discussion:
- the connections between 3 silk proteins : Fibl Fibh P25
- major proteins : H-chain, L-chain, P25
2011.07.15
Discussion:
- Due to limited resources and techniques required, the team decided to switch back to the previous project. Paper making !
- However it would be modified to be more innovative and creative.
2011.07.18
Discussion:
- Latest project : Photo paper
- cyanobacteria is designed as the host, cellulose made up of the glucose produced by cyanobacteria could be one of the main attraction of the project.
2011.07.23
Discussion:
- system modification to overcome the problems arises during preliminary round
- Biobricks from Tokyo 2010 team will be utilized
- regulator promoter in order to regulate the secretion of cellulose to solve the aggregation of the bacteria
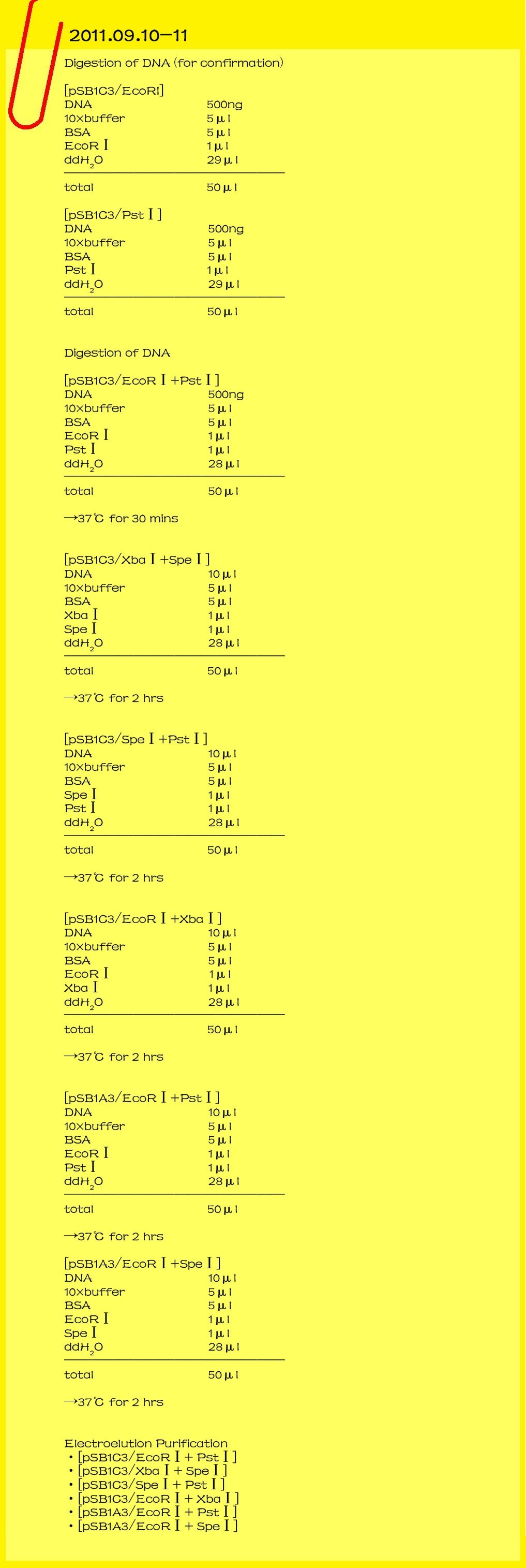
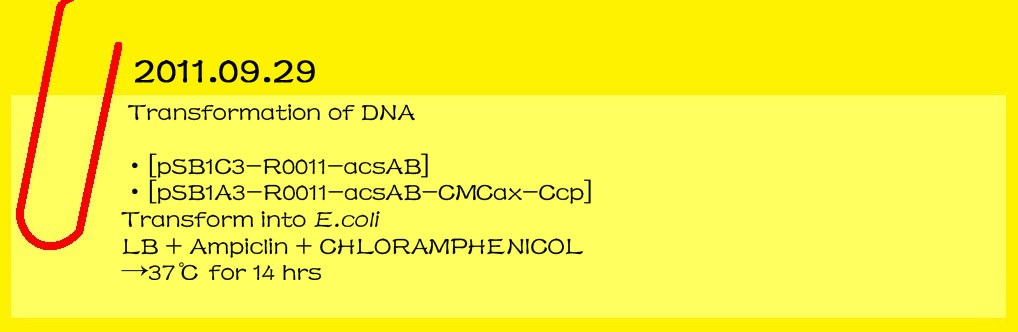
2011.09.15
Genome miniprep
Gluconacetobacter hansenii
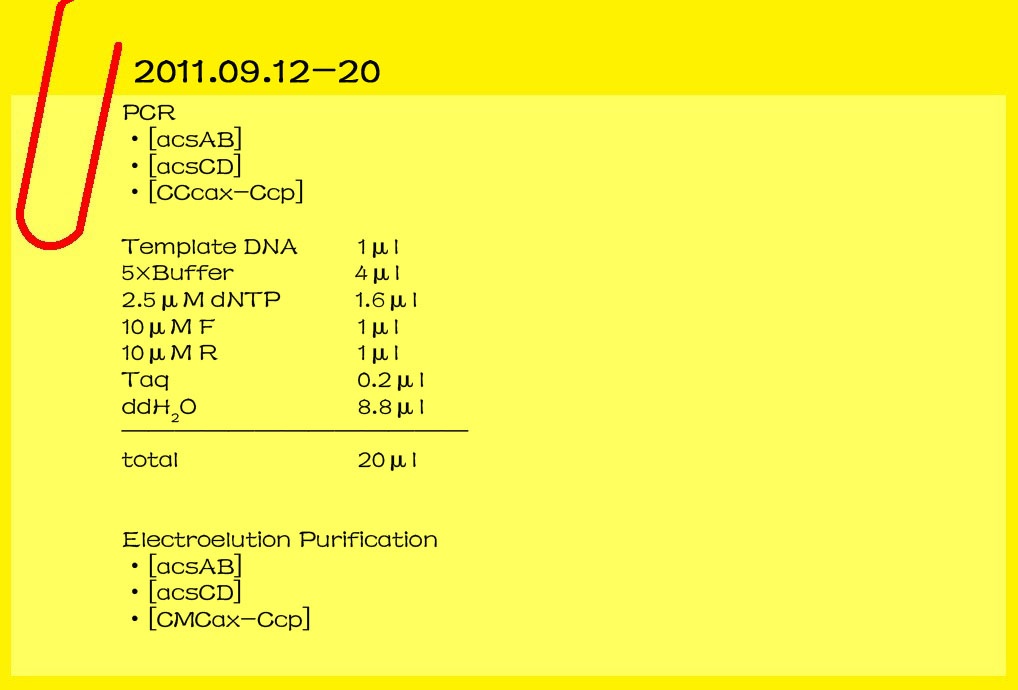
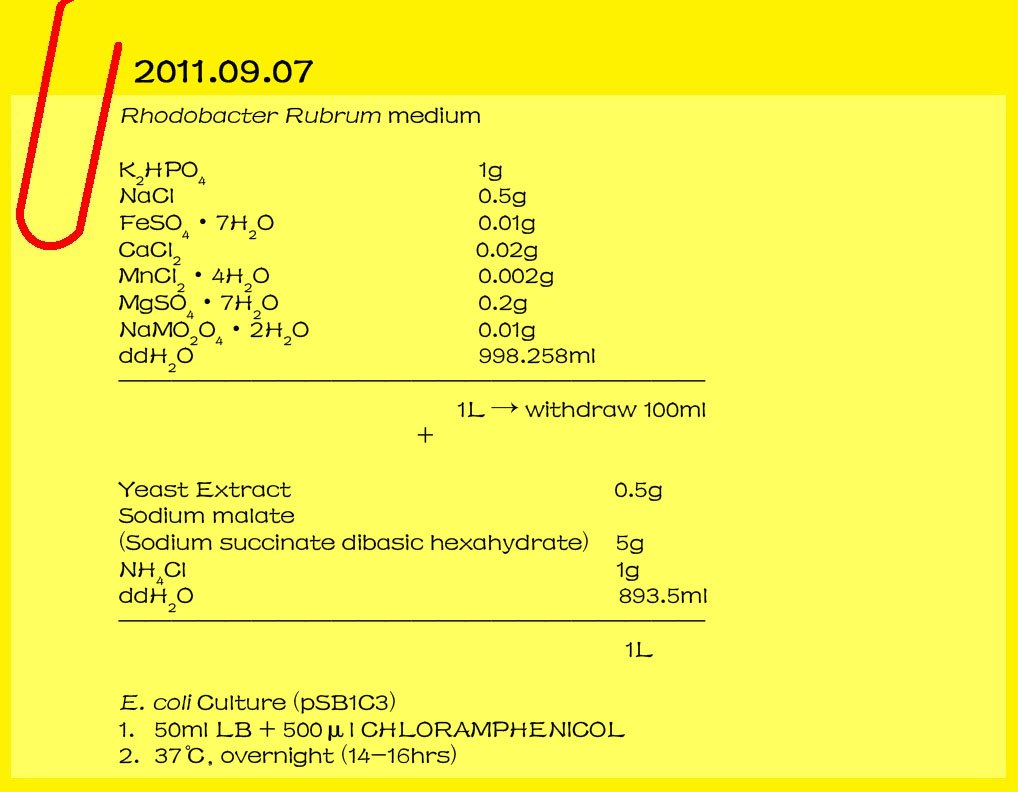
2011.09.18
Gel/PCR DNA extraction
Gluconacetobacter hansenii
Protocols



 "
"