|
|
| Line 69: |
Line 69: |
| | |- | | |- |
| | |style="width:900px;"|'''Week 4-5''' | | |style="width:900px;"|'''Week 4-5''' |
| - | <OL>
| + | tetO2 – 0 and tetO2 – 4 |
| - | <LI>tetO2 – 0 and tetO2 – 4
| + | |
| | <OL> | | <OL> |
| | <LI>Reverse PCR was used to insert tetO2 -0 and tetO2 – 4 into pEGFP-loxp-km-loxp. | | <LI>Reverse PCR was used to insert tetO2 -0 and tetO2 – 4 into pEGFP-loxp-km-loxp. |
| Line 77: |
Line 76: |
| | |- | | |- |
| | |style="width:900px;"|'''Week 6''' | | |style="width:900px;"|'''Week 6''' |
| - | <OL>
| + | Overlap PCR was carried out to produce fusion protein gene |
| - | <LI>Overlap PCR was carried out to produce fusion protein gene
| + | |
| | <OL> | | <OL> |
| | <LI>HNS::tetR | | <LI>HNS::tetR |
| Line 94: |
Line 92: |
| | </OL> | | </OL> |
| | </OL> | | </OL> |
| | + | |style="font-family: georgia, helvetica, arial, sans-serif;font-size:2em;color:#01DF01;"|Lab Diaries |
| | + | |- |
| | + | |style="width:900px;"|'''Week 7''' |
| | + | Overlap PCR was carried out to produce fusion protein gene tetR::HNS (2-90) |
| | + | <OL> |
| | + | <LI>The sticky ends of the enzyme products was transformed into blunt ends using PCR. |
| | + | |style="font-family: georgia, helvetica, arial, sans-serif;font-size:2em;color:#01DF01;"|Lab Diaries |
| | + | |- |
| | + | |style="width:900px;"|'''Week 8''' |
| | + | <OL> |
| | + | <LI>pROT-HNS (for BioBricks) was produced |
| | + | <LI>Double enzyme digestion was carried out to digest the fusion protein genes and ligate them to pBS1C3 (plasmid backbone) |
| | + | <OL> |
| | + | <LI>3 samples were transformed |
| | + | <OL> |
| | + | <LI>pSB1C3-tetR::HNS (2-83) |
| | + | <LI>pSB1C3-tetR::HNS (2-90) |
| | + | <LI>pSB1C3-tetR::HNS (FL) |
| | + | </OL> |
| | + | <LI>Enzyme digestion was carried out for the extracted and purified plasmid tetO2-0 and tetO2-3 |
| | + | <OL> |
| | + | <LI>0.5uL of CIP alkaline phosphatase was added to prevent self-ligation to each enzyme digest reaction mixture. |
| Lab Diaries
|
| Week 1
Transformation of reporter DNA (pEGFP-loxp-km-loxp) into DH10B (non-virulent strain E. coli) with antibiotic resistance (Chloramphenicol – Cm)
| Lab Diaries
|
Week 2
- tetO2 -1 (DNA binding site)
- Reverse PCR was used to insert tetO2 -1 into pEGFP-loxp-km-loxp (template DNA) by using forward and reverse primers which are with the tetO2 -1. By using reverse PCR, we can insert the tetO2 -1 site into the pEGFP while the plasmid produced is still in double-stranded circular form.
- pEGFP-loxp-km-loxp-tetO2-1 was then transformed into DH10B to greatly amplify the product by using bacterial cells.
- tetO2 -2 (DNA biding site)
- Reverse PCR was used to insert tetO2 -2 into pEGFP-loxp-km-loxp.
- 2uL of 10-fold diluted pEGFP-loxp-km-loxp- tetO2 -2 was transformed into DH10B to greatly amplify the product by using bacterial cells.
- tetO2 – 3 (DNA binding site)
- Reverse PCR was used to insert tetO2 – 3 into pEGFP-loxp-km-loxp.
- 2uL of 10-fold diluted pEGFP-loxp-km-loxp- tetO2 – 3 was transformed into DH10B to greatly amplify the product by using bacterial cells.
| Lab Diaries
|
Week 3
- Overlap PCR (involving 3 steps) was carried out to produce the fusion protein [tetR-HNS (any length)].
- PCR was carried out separately for 2 genes, tetR and HNS (full length, in this case).
- Primers used were as below:
- tetR: [forward] R-H-out-F; [reverse] R-H-tetR-R
- HNS (FL): [forward] R-H-tetR-F-out; [reverse] R-H(FL)-2-R
|
| Fusion protein genes produced separately using PCR
|
- Another PCR was carried out using primers with linker to insert the linker DNA to the tetR and HNS (FL) for the fusion of the 2 genes.
- PCR was used to further amplify the product from step 2 (fusion protein gene).
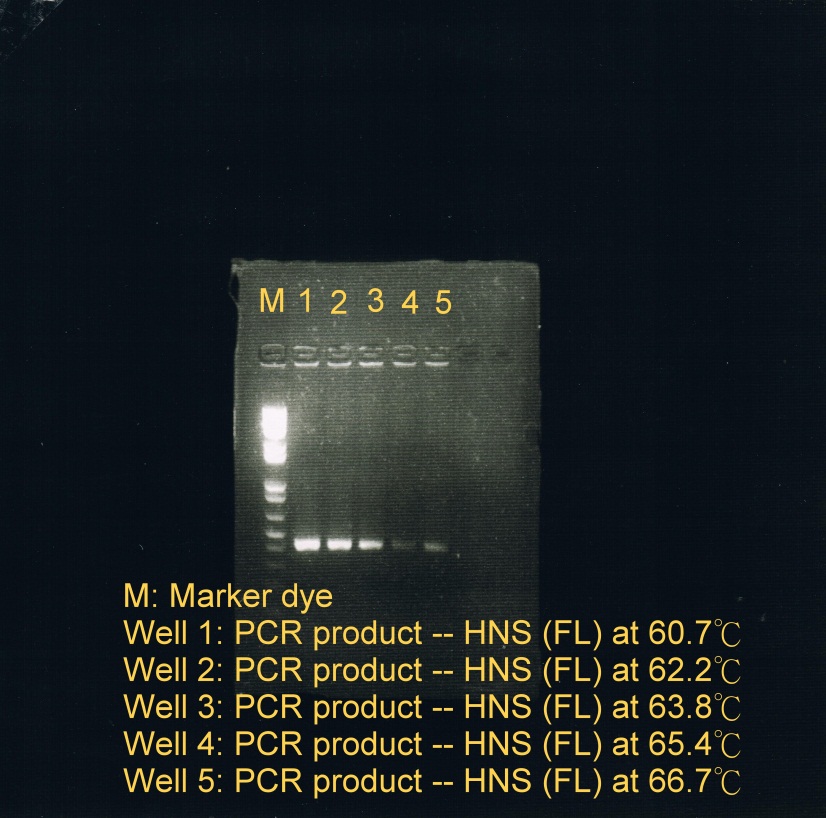
- PCR was used to obtain HNS(FL) at 5 different temperatures to test which temperature is optimal for annealing.
- Annealing phase at 5 different temperatures:
- 60.7C
- 62.2C
- 63.8C
- 65.4C
- 66.7C (less sample)
- Electrophoresis was carried to determine which temperature best suit the annealing phase. From the gel image, it was observed that a high concentration of products was resulted at annealing temperature between 60.7C and 62.2C.
|
| Fusion protein genes annealed at 5 different temperatures
|
| Lab Diaries
|
| Week 4-5
tetO2 – 0 and tetO2 – 4
- Reverse PCR was used to insert tetO2 -0 and tetO2 – 4 into pEGFP-loxp-km-loxp.
- 2uL of pEGFP-loxp-km-loxp- tetO2 – 0 and pEGFP-loxp-km-loxp- tetO2 – 4 were transformed into DH10B separately to greatly amplify the product by using bacterial cells.
| Lab Diaries
|
| Week 6
Overlap PCR was carried out to produce fusion protein gene
- HNS::tetR
- HNS (1-46)
- HNS (1-83)
- HNS (1-90)
- HNS (FL)
- tetR::HNS
- HNS (2-46)
- HNS (2-83)
- HNS (FL)
| Lab Diaries
|
| Week 7
Overlap PCR was carried out to produce fusion protein gene tetR::HNS (2-90)
- The sticky ends of the enzyme products was transformed into blunt ends using PCR.
| Lab Diaries
|
Week 8
- pROT-HNS (for BioBricks) was produced
- Double enzyme digestion was carried out to digest the fusion protein genes and ligate them to pBS1C3 (plasmid backbone)
- 3 samples were transformed
- pSB1C3-tetR::HNS (2-83)
- pSB1C3-tetR::HNS (2-90)
- pSB1C3-tetR::HNS (FL)
- Enzyme digestion was carried out for the extracted and purified plasmid tetO2-0 and tetO2-3
- 0.5uL of CIP alkaline phosphatase was added to prevent self-ligation to each enzyme digest reaction mixture.
|
 "
"




