Team:HKU-Hong Kong/Modelling
From 2011.igem.org
(Difference between revisions)
| Line 14: | Line 14: | ||
|- | |- | ||
|TetR | |TetR | ||
| - | |||
</div> | </div> | ||
| + | |} | ||
{| style="width:900px;background:#000000;text-align:justify;font-family: georgia, helvetica, arial, sans-serif;color:#ffffff;margin-top:25px;" cellspacing="20" | {| style="width:900px;background:#000000;text-align:justify;font-family: georgia, helvetica, arial, sans-serif;color:#ffffff;margin-top:25px;" cellspacing="20" | ||
|style="width:900px;"| | |style="width:900px;"| | ||
| Line 30: | Line 30: | ||
</div> | </div> | ||
|} | |} | ||
| + | |||
| + | {| style="width:900px;background:#000000;text-align:justify;font-family: georgia, helvetica, arial, sans-serif;color:#ffffff;margin-top:25px;" cellspacing="20" | ||
| + | |style="width:900px;"| | ||
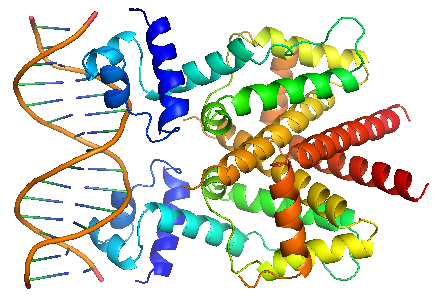
| + | TetR binds with tetO under normal circumstances. In the DNA-bound complex, the two-fold symmetry of TetR is maintained. Each HTH-motif binds to the corresponding major groove of the palindromic tetO, while the minor groove is not recognized. All but the central 3 pairs of the 15-mer operator fragment are engaged in the binding. | ||
| + | In the presence of tetracycline – magnesium complex, [MgTc]+, residue His100 and Thr103 of helix α6 are displaced, causing the shifting of helix α6 towards the C terminal. Both C and N terminal of α4 shift because of the movement of α6 and the formation of hydrogen bond between α4 and [MgTc]+. α3 is displaced as a consequence, which releases the bonding between tetR and tetO.<br/> | ||
| + | |} | ||
| + | |||
| + | {| style="width:900px;background:#000000;text-align:justify;font-family: georgia, helvetica, arial, sans-serif;color:#ffffff;margin-top:25px;" cellspacing="20" | ||
| + | |style="width:900px; font-size:1.5em;color:#CC0000;"|DNA-binding protein H-NS | ||
| + | |- | ||
| + | |style="width:900px;"| | ||
| + | H-NS proteins are a type of histone-like protein found in abundance in Gram-negative bacteria (such as E.coli). Phylogenetic studies have shown there are some varieties of H-NS proteins in different bacteria species. Nonetheless, H-NS are involved in regulating transcription by repression, and the structuring of the nucleoid. The regulation of H-NS expression is complex. Its expression is likely to be influenced by the external environment as well. However, it is also capable of autoregulating its own transcriptions. | ||
| + | H-NS regulates gene expression negatively; whilst a complete understanding of its mechanism is yet to be established due to limitations of current lab techniques, research has identified a number of common themes: first, H-NS proteins acts on (non-specifically) intrinsically curved DNA sites near the promoter of the gene to be repressed. Intrinsically curved DNA sites near the promoter enable upstream and downstream DNA to be brought in close proximity after the binding of the RNA polymerase on the promoter. Bound H-NS on these regions interacts to form a nucleoprotein complex that traps RNA polymerase, thus effectively stopping transcription. The trapping of the RNA polymerase on the promoter means it need not be recruited from the cytoplasm again once repression is relieved, hence allowing a rapid response to changes in environmental conditions. | ||
| + | |- | ||
| + | <div ALIGN=CENTER> | ||
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:TetR.png|250px]] | ||
| + | |- | ||
| + | |TetR | ||
| + | </div> | ||
| + | |} | ||
Revision as of 10:36, 4 October 2011
 "
"