Team:UTP-Panama/Week 12
From 2011.igem.org
(→WET LAB) |
(→WET LAB) |
||
| (3 intermediate revisions not shown) | |||
| Line 46: | Line 46: | ||
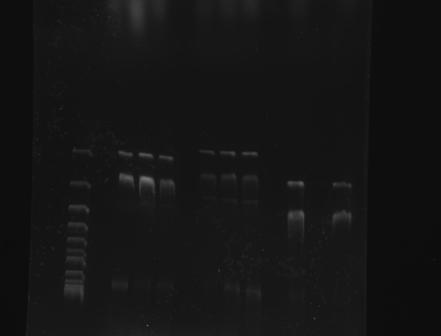
Gel Electrophoresis of Part:BBa_K410000 and the CspA Promoter <br> | Gel Electrophoresis of Part:BBa_K410000 and the CspA Promoter <br> | ||
| - | + | 1 2 3 4 5 6 7 8 9 10 | |
[[File:GATECH, UNAM 003 Y 001 TOMA2 24AGO11.jpg]] | [[File:GATECH, UNAM 003 Y 001 TOMA2 24AGO11.jpg]] | ||
| + | |||
| + | 1. Reference (DNA Supercoiled Ladder) | ||
| + | |||
| + | 2, 3, 4 : BBa_K328001 | ||
| + | |||
| + | 5, 6, 7 : BBa_K328003 | ||
| + | |||
| + | 8, 9, 10: BBa_K410000 | ||
==August 27== | ==August 27== | ||
Latest revision as of 04:57, 29 September 2011
|
Home |
Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | Week 16 | Week 17 | After Regional Week 1 | After Regional Week 2 |
Week 12: August 22 to 27August 22WET LABPreparation of LB We prepared additional 15 mL LB to continue activities from Aug 19, 2011
calculation: August 23WET LABWe did a dirty miniprep to extract plasmidic DNA of the Biobricks August 24WET LABElectrophoresis was applied to the following BioBricks: • BBa_K410000, pSB1C3 1. Preparation of agarose 1%: Gel Electrophoresis of Part:BBa_K410000 and the CspA Promoter 1 2 3 4 5 6 7 8 9 10 1. Reference (DNA Supercoiled Ladder) 2, 3, 4 : BBa_K328001 5, 6, 7 : BBa_K328003 8, 9, 10: BBa_K410000 August 27GENERAL MEETING SESSIONSAFETY DUE HUMAN PRACTICE We also prepared details of our project and ongoing activies. Director of Session: Grimaldo E. Ureña & Lucia Palma. |
 "
"