Team:Lethbridge/Results
From 2011.igem.org
Liszabruder (Talk | contribs) |
Liszabruder (Talk | contribs) |
||
| Line 50: | Line 50: | ||
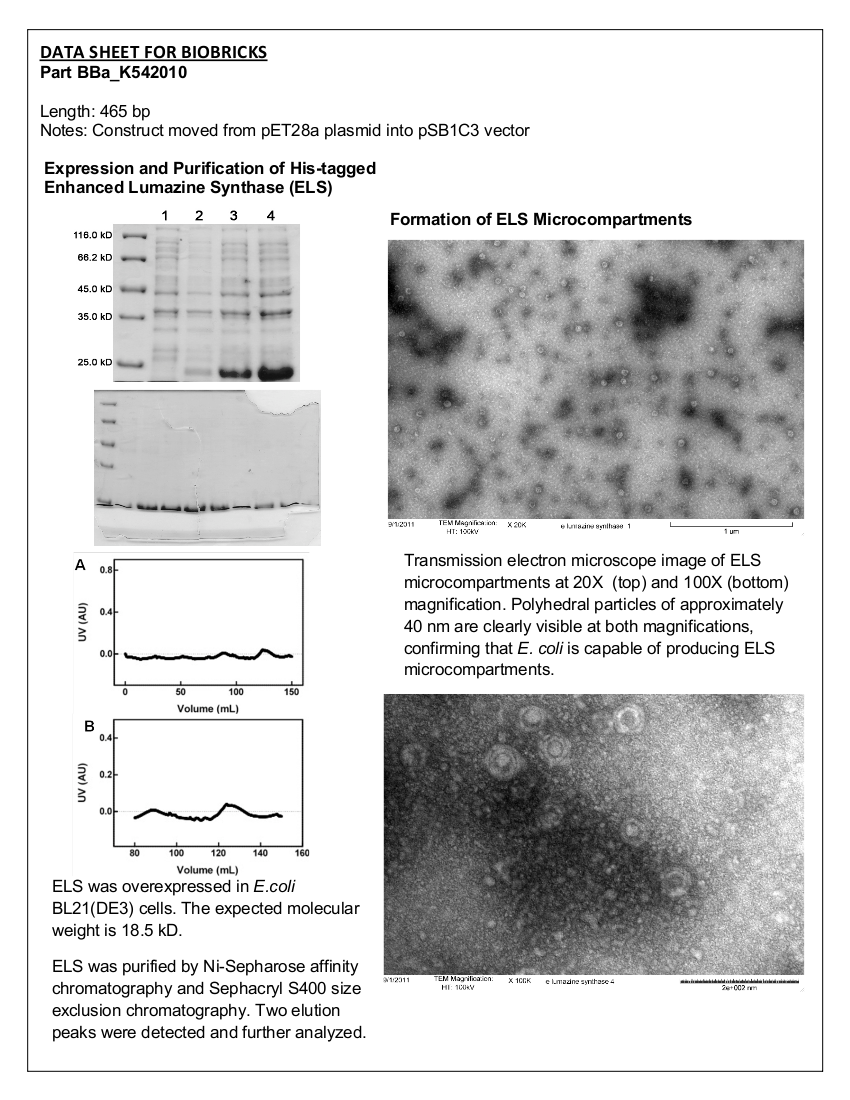
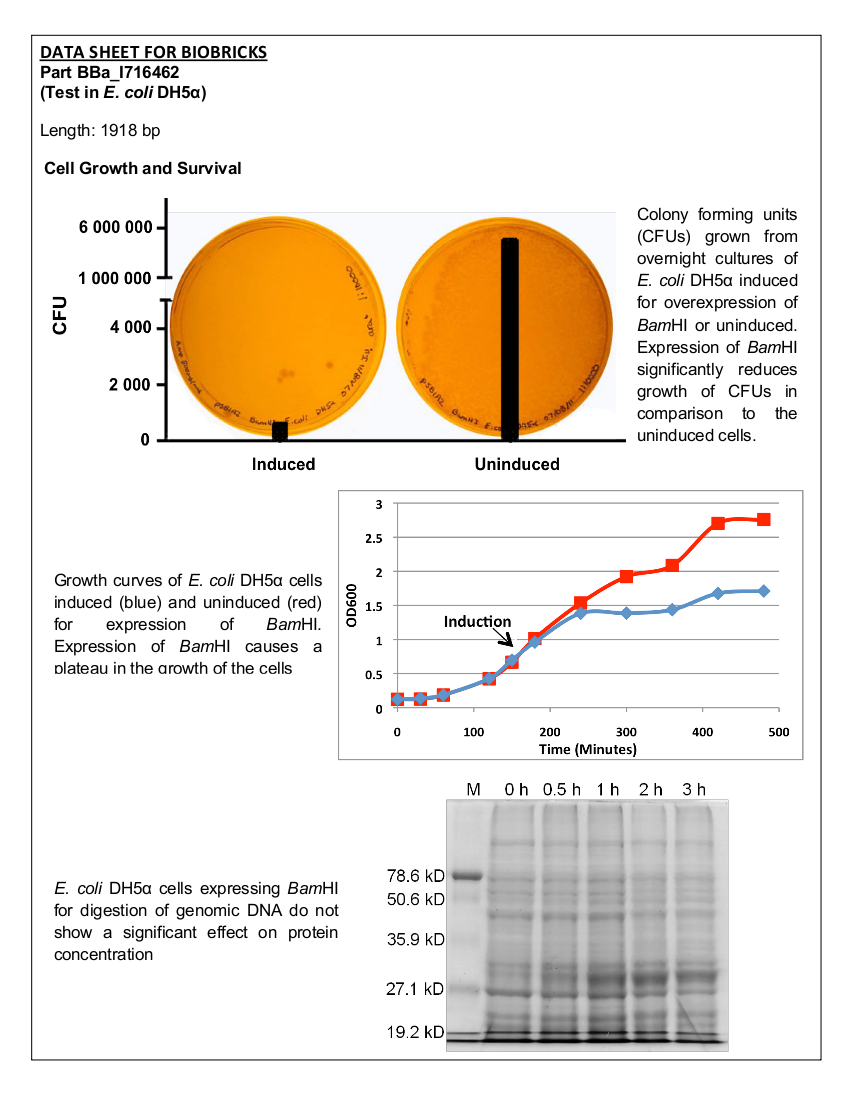
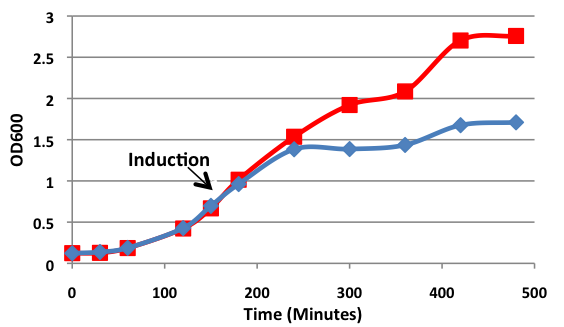
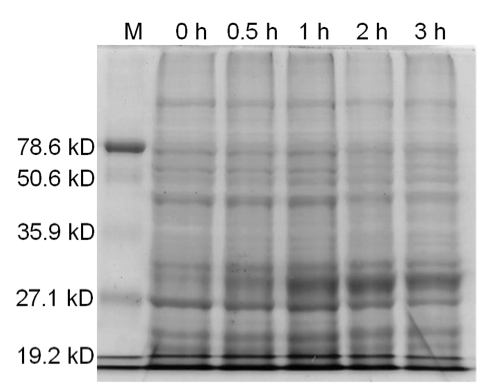
4 X 500 mL of LB media in 2 L flasks were inoculated with <i>E. coli</i> BL21 (DE3) cells expressing ELS. Cells were grown at 37°C with shaking until the culture reached an OD<sub>600</sub> of 0.683. The cultures were then induced with 10 mM IPTG. Culture samples containing equal amounts of cells were taken before induction and 30 min, 1 hour, 2 hours, and 3 hours post induction for analysis by SDS-PAGE. At 5 hours post induction the cells were harvested by centrifugation at 5000 x g for 5 min. The cell lysate was resuspended in 42 mL of buffer containing 50 mM Tris pH 8.0, 60 mM NH<sub>4</sub>CL, 7 mM β-mercaptoethanol, 1 mM PMSF, 7 mM MgCl<sub>2</sub>, 300 mM KCl, 10 mM imidazole, and 15% glycerol. Afterwards, 0.05 g of crystallized lysozyme was added to the cell suspension and incubated on ice for 1 hour. The lysate was then centrifuged for 70 minutes at 30000 x g to obtain the S30 fraction. | 4 X 500 mL of LB media in 2 L flasks were inoculated with <i>E. coli</i> BL21 (DE3) cells expressing ELS. Cells were grown at 37°C with shaking until the culture reached an OD<sub>600</sub> of 0.683. The cultures were then induced with 10 mM IPTG. Culture samples containing equal amounts of cells were taken before induction and 30 min, 1 hour, 2 hours, and 3 hours post induction for analysis by SDS-PAGE. At 5 hours post induction the cells were harvested by centrifugation at 5000 x g for 5 min. The cell lysate was resuspended in 42 mL of buffer containing 50 mM Tris pH 8.0, 60 mM NH<sub>4</sub>CL, 7 mM β-mercaptoethanol, 1 mM PMSF, 7 mM MgCl<sub>2</sub>, 300 mM KCl, 10 mM imidazole, and 15% glycerol. Afterwards, 0.05 g of crystallized lysozyme was added to the cell suspension and incubated on ice for 1 hour. The lysate was then centrifuged for 70 minutes at 30000 x g to obtain the S30 fraction. | ||
====Results==== | ====Results==== | ||
| - | [[image:uoflELSexpressiongel.png|center]] | + | [[image:uoflELSexpressiongel.png|center|400px]] |
<br><br> | <br><br> | ||
<b>Figure 1.</b> 12.5% SDS-PAGE of His-tagged ELS Overexpressed in <i>E. coli</i> BL21 (DE3). Samples were taken at 1 hour increments after IPTG induction. Lane 1: zero hours; Lane 2: 1 hour; Lane 3: 2 hours; Lane 4: 3 hours. The expected molecular weight of enhanced lumazine synthase is 18.5 kD. | <b>Figure 1.</b> 12.5% SDS-PAGE of His-tagged ELS Overexpressed in <i>E. coli</i> BL21 (DE3). Samples were taken at 1 hour increments after IPTG induction. Lane 1: zero hours; Lane 2: 1 hour; Lane 3: 2 hours; Lane 4: 3 hours. The expected molecular weight of enhanced lumazine synthase is 18.5 kD. | ||
| Line 64: | Line 64: | ||
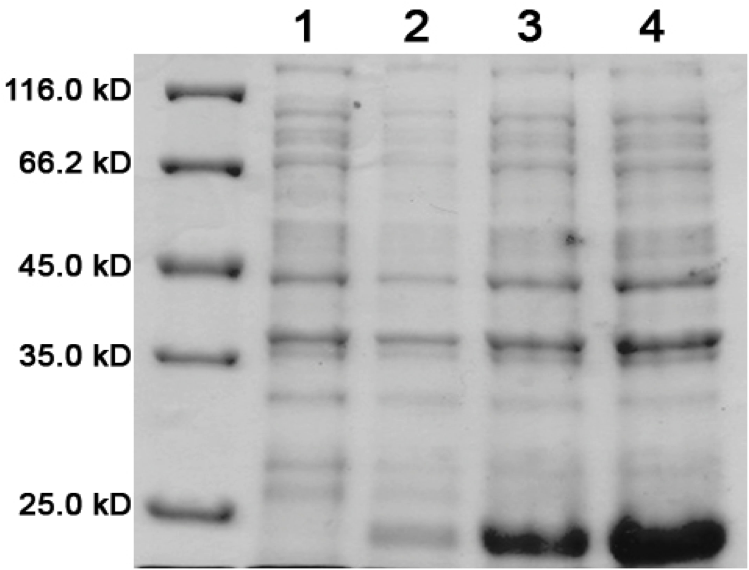
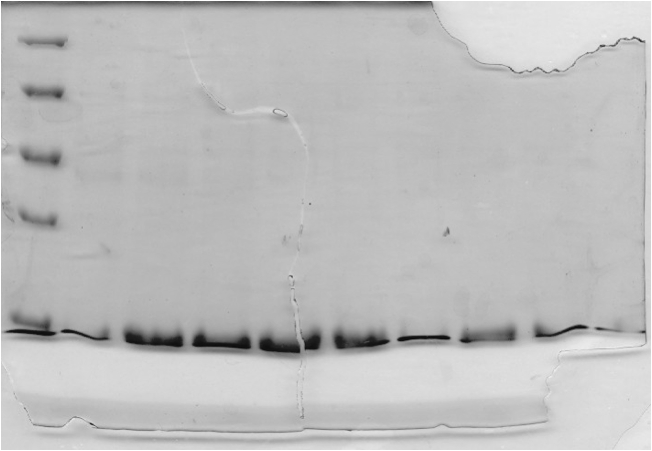
Concentrated protein samples from the Ni<sup>2+</sup>-Sepharose column were applied to a Sephacryl S400 size exclusion chromatography column at a flow rate of 0.4 mL/minute. 150 mL of SEC Buffer (50 mM sodium phosphate, 5 mM EDTA, 200 mM NaCl, pH 8.0, with 20% glycerol) was pumped through the column. The fractions eluted were collected and the absorbance was measured at 280 nm (see Fig 3 and 4). | Concentrated protein samples from the Ni<sup>2+</sup>-Sepharose column were applied to a Sephacryl S400 size exclusion chromatography column at a flow rate of 0.4 mL/minute. 150 mL of SEC Buffer (50 mM sodium phosphate, 5 mM EDTA, 200 mM NaCl, pH 8.0, with 20% glycerol) was pumped through the column. The fractions eluted were collected and the absorbance was measured at 280 nm (see Fig 3 and 4). | ||
====Results==== | ====Results==== | ||
| - | [[image:uoflELSsdspageimac.png|center]] | + | [[image:uoflELSsdspageimac.png|center|400px]] |
<br> | <br> | ||
<b>Figure 2.</b> SDS-PAGE (15%) of ELS purification by IMAC. Lane 1: protein marker. Lanes 2-10: Elution Fractions 1 – 9. | <b>Figure 2.</b> SDS-PAGE (15%) of ELS purification by IMAC. Lane 1: protein marker. Lanes 2-10: Elution Fractions 1 – 9. | ||
<br><br> | <br><br> | ||
| - | [[image:uoflELSchromatograms.png|center]] | + | [[image:uoflELSchromatograms.png|center|600px]] |
<br> | <br> | ||
<b>Figure 3.</b> (A) Concentrated protein sample from the Ni<sup>2+</sup>-Sepharose column applied to a Sephacryl S400 size exclusion chromatography column at a flow rate of 0.4 mL/minute. The absorbance of the eluting solution was measured at 280nm. (B) Magnified view of chromatogram of the two peaks seen in (A). | <b>Figure 3.</b> (A) Concentrated protein sample from the Ni<sup>2+</sup>-Sepharose column applied to a Sephacryl S400 size exclusion chromatography column at a flow rate of 0.4 mL/minute. The absorbance of the eluting solution was measured at 280nm. (B) Magnified view of chromatogram of the two peaks seen in (A). | ||
| Line 80: | Line 80: | ||
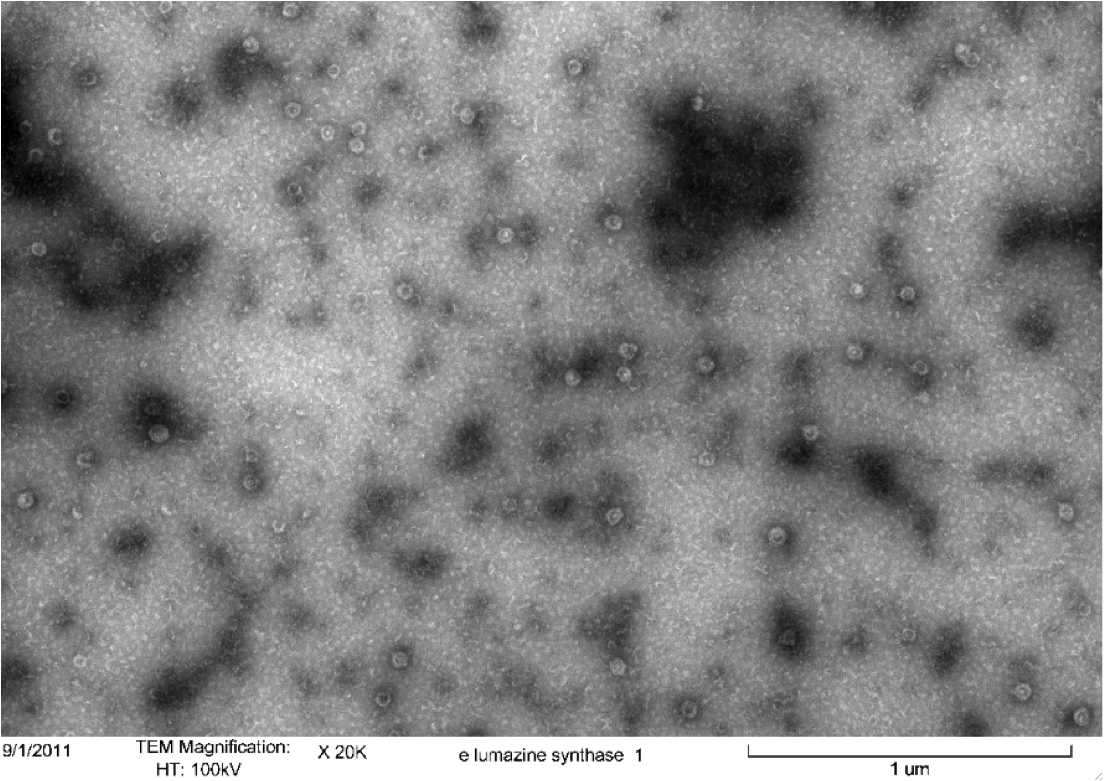
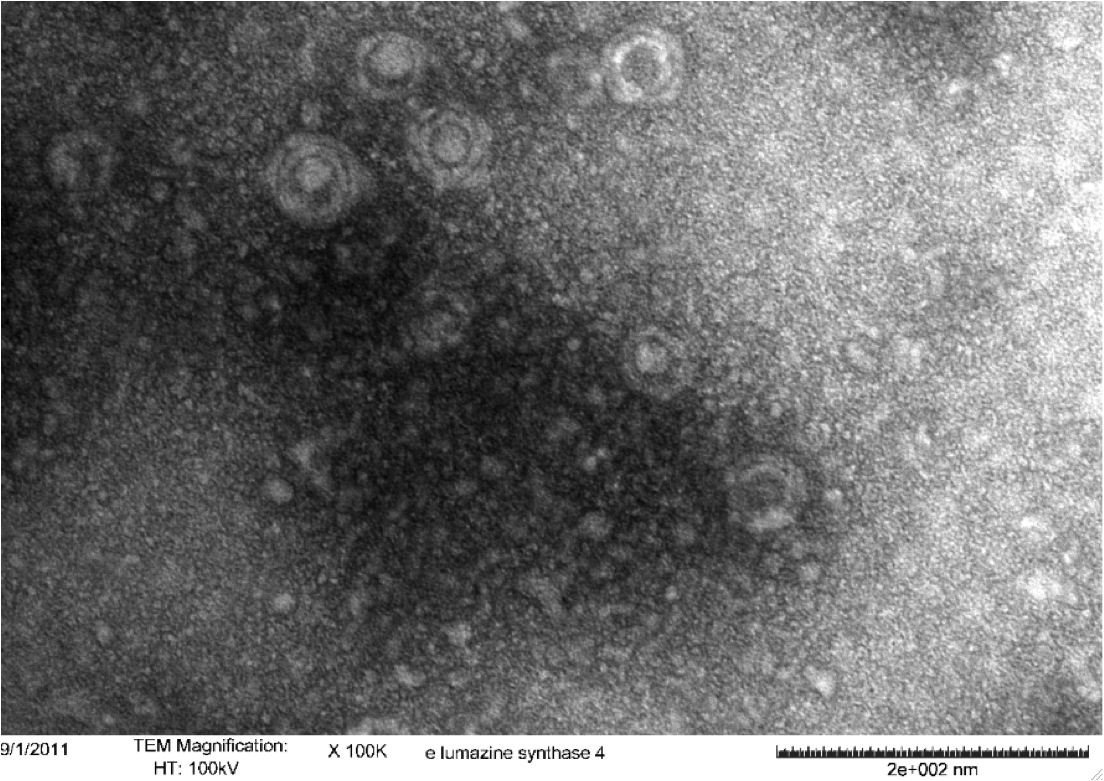
Purified Samples in solution were placed on a carbon grid and negatively stained using uranyl acetate. Carbon grids containing the samples were then viewed with a Hitachi H-7500 Transmission Electron Microscope. | Purified Samples in solution were placed on a carbon grid and negatively stained using uranyl acetate. Carbon grids containing the samples were then viewed with a Hitachi H-7500 Transmission Electron Microscope. | ||
====Results==== | ====Results==== | ||
| - | [[image:uoflELSTEM1.png|center]] | + | [[image:uoflELSTEM1.png|center|400px]] |
<br> | <br> | ||
<b>Figure 4.</b> Transmission electron microscopy of Enhanced Lumazine Synthase microcompartments. Microcompartments of approximately 40 nm can be seen at x 20K magnification. | <b>Figure 4.</b> Transmission electron microscopy of Enhanced Lumazine Synthase microcompartments. Microcompartments of approximately 40 nm can be seen at x 20K magnification. | ||
<br><br> | <br><br> | ||
| - | [[image: | + | [[image:uoflELSTEM2.png|center|400px]] |
<br> | <br> | ||
<b>Figure 5.</b> Transmission electron microscopy of Enhanced Lumazine Synthase microcompartments. Microcompartments of approximately 40 nm can be seen at x 100K magnification. | <b>Figure 5.</b> Transmission electron microscopy of Enhanced Lumazine Synthase microcompartments. Microcompartments of approximately 40 nm can be seen at x 100K magnification. | ||
Revision as of 16:29, 28 September 2011
|
|
|
|---|
 "
"