Team:UC Davis/Notebook/Week 3
From 2011.igem.org
(Created page with "== Week 3 == --Monday-- Today we made Dh5a competent cells as we had run out of our stocks. Keegan made a Gibson volume calculator which will easily calculate the volumes of ea...") |
|||
| (16 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | == Week 3 == | + | {{Team:UC_Davis/Head}} |
| + | |||
| + | <html> | ||
| + | <style> | ||
| + | |||
| + | #navcon { | ||
| + | background-image:url('https://static.igem.org/mediawiki/2011/4/47/UCD_paperheadersketch.JPG'); | ||
| + | } | ||
| + | </style> | ||
| + | <script> | ||
| + | thispagenumber = 4; | ||
| + | </script> | ||
| + | </html> | ||
| + | |||
| + | <html> | ||
| + | <div class="floatbox"> | ||
| + | <center> | ||
| + | <h3>Week Selection</h3> | ||
| + | <table id="linktable"> | ||
| + | <tr> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_1">1</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_2">2</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_3">3</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_4">4</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_5">5</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_6">6</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_7">7</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_8">8</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_9">9</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_10">10</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_11">11</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_12">12</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_13">13</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_14">14</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_15">15</a></td> | ||
| + | <td><a href="https://2011.igem.org/Team:UC_Davis/Notebook/Week_16">16</a></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | </center> | ||
| + | </div> | ||
| + | </html> | ||
| + | |||
| + | |||
| + | <html> | ||
| + | <div class="floatbox"> | ||
| + | |||
| + | <html><div class ="floatbox3"> | ||
| + | <img src="https://static.igem.org/mediawiki/2011/3/30/UCD_Week3_banner.JPG" align="left" width="300" height="143"> | ||
| + | |||
| + | A lot of detective work went into this week, through which we discovered that many of our recent transformations have been failing because of accidentally making LB+chloro plates instead of LB+carb plates. After resolving the issue, we reattempted to transform our LacI promoter mutants in the promoter screening plasmid and continued with construction. We also began work with our new bw22826 strain, which will be very useful to us when we start characterizing the mutants. | ||
| + | |||
| + | </div></div> | ||
| + | |||
| + | <div class="floatbox"> | ||
| + | </html> | ||
--Monday-- | --Monday-- | ||
| - | Today we made Dh5a competent cells as we had run out of our stocks. Keegan made a Gibson volume calculator which will easily calculate the volumes of each part you need when you input the concentrations of those parts to make sure you have equal molar amounts. It will be handy for quick calculations of assemblies of up to 10 parts at a time. | + | Today we made Dh5a [[Team:UC_Davis/Protocols#compcell|competent cells]] as we had run out of our stocks. Keegan made a Gibson volume calculator which will easily calculate the volumes of each part you need when you input the concentrations of those parts to make sure you have equal molar amounts. It will be handy for quick calculations of assemblies of up to 10 parts at a time. |
<html> | <html> | ||
| - | <img src=" | + | <img src="http://farm7.static.flickr.com/6158/6178348257_a3b6fc291f.jpg" width="600" height="350"> |
</html> | </html> | ||
| + | <html> | ||
| + | </div> | ||
| + | <div class="floatbox"> | ||
| + | </html> | ||
--Tuesday-- | --Tuesday-- | ||
| - | Today we transformed the LacI promoter mutants into the screening plasmid Bba_J61002. We're hoping to see red from the RFP that is built into the screening plasmid. Ideally we will see shades of red corresponding to differing strengths of the mutant promoters. Although we're planning on using a fluorescent cell sorter, a cursory screening could be useful just to make sure our error-prone PCR was successful. We transformed into Dh5a E. coli so we had to induce our plates with IPTG since this particular strain is LacI+. | + | Today we [[Team:UC_Davis/Protocols#transformation|transformed]] the LacI promoter [[Team:UC_Davis/Protocols#errprone|mutants]] into the screening plasmid Bba_J61002. We're hoping to see red from the RFP that is built into the screening plasmid. Ideally we will see shades of red corresponding to differing strengths of the mutant promoters. Although we're planning on using a fluorescent cell sorter, a cursory screening could be useful just to make sure our [[Team:UC_Davis/Protocols#errprone|error-prone PCR]] was successful. We [[Team:UC_Davis/Protocols#transformation|transformed]] into Dh5a E. coli so we had to induce our plates with IPTG since this particular strain is LacI+. |
We had some very unusual weather here today. Normal Davis summers are very hot and dry and today should have been around 90-100F but it was rainy and cold! Global climate change perhaps? | We had some very unusual weather here today. Normal Davis summers are very hot and dry and today should have been around 90-100F but it was rainy and cold! Global climate change perhaps? | ||
| + | <html> | ||
| + | </div> | ||
| + | <div class="floatbox"> | ||
| + | </html> | ||
--Wednesday-- | --Wednesday-- | ||
<html> | <html> | ||
| - | <img src=" | + | <img src="http://farm7.static.flickr.com/6160/6178348911_f6c530acd3.jpg" width="350" height="550"> |
</html> | </html> | ||
| - | We realized today that we needed to test some carb plates that we made last week. Our suspicions arose when 16 ligations that had previously been transformed successfully failed to grow on our new plates. We did a quick test by streaking a couple plates with a part in an AK backbone. We streaked the same part on both Kan and Carb plates and only the Kan plates had any growth on them. We will make some new Carb plates tomorrow and hopefully they'll work! Today we also designed the rest of our Gibson assembly primers for constructing our screening plasmid. We'll order tomorrow and in about a week be able to assemble everything fairly quickly. In the meantime we'll continue using biobrick assembly. | + | We realized today that we needed to test some carb plates that we made last week. Our suspicions arose when 16 [[Team:UC_Davis/Protocols#ligate|ligations]] that had previously been [[Team:UC_Davis/Protocols#transformation|transformed]] successfully failed to grow on our new plates. We did a quick test by streaking a couple plates with a part in an AK backbone. We streaked the same part on both Kan and Carb plates and only the Kan plates had any growth on them. We will make some new Carb plates tomorrow and hopefully they'll work! Today we also designed the rest of our Gibson assembly primers for constructing our screening plasmid. We'll order tomorrow and in about a week be able to assemble everything fairly quickly. In the meantime we'll continue using biobrick assembly. |
| + | <html> | ||
| + | </div> | ||
| + | <div class="floatbox"> | ||
| + | </html> | ||
--Thursday-- | --Thursday-- | ||
| - | We did PCR of E0240 with and without B0034. We wrote our formal letter to Novozymes and set up digest with E and P of E0240 modified with and without B0034. We also got a new strain of E. coli, BW22826 which is F-, Δ(araD-araB)567, Δ(codB-lacI)3, &lambda-, rpoS396(Am), rph-1. This strain will be helpful for us since it is both araBAD and LacI negative. This means we won't have to put an arabinose repressor in our plasmid which will cut down on construction time and allow for more testing time! We also needed the strain to be ∆LacI since we will be testing the Lac repressor and operator. | + | We did [[Team:UC_Davis/Protocols#PCR|PCR]] of E0240 with and without B0034. We wrote our formal letter to Novozymes and set up [[Team:UC_Davis/Protocols#digest|digest]] with E and P of E0240 modified with and without B0034. We also got a new strain of E. coli, BW22826 which is F-, Δ(araD-araB)567, Δ(codB-lacI)3, &lambda-, rpoS396(Am), rph-1. This strain will be helpful for us since it is both araBAD and LacI negative. This means we won't have to put an arabinose repressor in our plasmid which will cut down on construction time and allow for more testing time! We also needed the strain to be ∆LacI since we will be testing the Lac repressor and operator. |
| - | We also made carbenicillin plates and diagnosed the "bad" carb plates as chloramphenocol plates. Our mistake came when we used an older stock of carb which was labelled only with a 'c'. Lesson learned; don't assume anything! | + | We also made carbenicillin plates and diagnosed the "bad" carb plates as chloramphenocol plates. Our mistake came when we used an older stock of "carb" which was labelled only with a 'c'. Lesson learned; don't assume anything! |
| + | <html> | ||
| + | </div> | ||
| + | <div class="floatbox"> | ||
| + | </html> | ||
--Friday-- | --Friday-- | ||
| Line 34: | Line 104: | ||
<html> | <html> | ||
| - | <img src=" | + | <img src="http://farm7.static.flickr.com/6152/6178872686_806975d2ac.jpg" width="600" height="350"> |
</html> | </html> | ||
| + | <html> | ||
| + | </div> | ||
| + | <div class="floatbox"> | ||
| + | </html> | ||
--Saturday-- | --Saturday-- | ||
Took plates out and all had growth=carb plates good! It's amazing what plating on the right antibiotic will do. All of our plates which had failed just a few days ago had colonies with a few of them being red. | Took plates out and all had growth=carb plates good! It's amazing what plating on the right antibiotic will do. All of our plates which had failed just a few days ago had colonies with a few of them being red. | ||
| + | |||
| + | <html> | ||
| + | |||
| + | <style> | ||
| + | |||
| + | h1{ | ||
| + | color:#EE3333; | ||
| + | font-size: 40px; | ||
| + | } | ||
| + | h2{ | ||
| + | color:#EE3333; | ||
| + | font-size: 28px; | ||
| + | } | ||
| + | h3{ | ||
| + | color:#EE3333; | ||
| + | font-size: 20px; | ||
| + | } | ||
| + | |||
| + | |||
| + | |||
| + | </style> | ||
| + | |||
| + | <script> | ||
| + | $('#linktable td').mouseover(function(){ | ||
| + | $(this).dequeue().animate({opacity:1}); | ||
| + | }); | ||
| + | $('#linktable td').mouseout(function(){ | ||
| + | $(this).dequeue().animate({opacity:.3}); | ||
| + | }); | ||
| + | </script> | ||
| + | |||
| + | |||
| + | |||
| + | </html> | ||
Latest revision as of 07:22, 28 September 2011
Start a Family
Got a favorite BioBrick? Check our our process for expanding basic parts into part families.Criteria
View our judging criteria for iGEM 2011 here.
 A lot of detective work went into this week, through which we discovered that many of our recent transformations have been failing because of accidentally making LB+chloro plates instead of LB+carb plates. After resolving the issue, we reattempted to transform our LacI promoter mutants in the promoter screening plasmid and continued with construction. We also began work with our new bw22826 strain, which will be very useful to us when we start characterizing the mutants.
A lot of detective work went into this week, through which we discovered that many of our recent transformations have been failing because of accidentally making LB+chloro plates instead of LB+carb plates. After resolving the issue, we reattempted to transform our LacI promoter mutants in the promoter screening plasmid and continued with construction. We also began work with our new bw22826 strain, which will be very useful to us when we start characterizing the mutants.
Today we made Dh5a competent cells as we had run out of our stocks. Keegan made a Gibson volume calculator which will easily calculate the volumes of each part you need when you input the concentrations of those parts to make sure you have equal molar amounts. It will be handy for quick calculations of assemblies of up to 10 parts at a time.
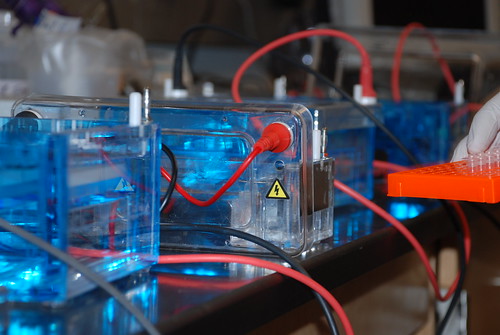
Today we transformed the LacI promoter mutants into the screening plasmid Bba_J61002. We're hoping to see red from the RFP that is built into the screening plasmid. Ideally we will see shades of red corresponding to differing strengths of the mutant promoters. Although we're planning on using a fluorescent cell sorter, a cursory screening could be useful just to make sure our error-prone PCR was successful. We transformed into Dh5a E. coli so we had to induce our plates with IPTG since this particular strain is LacI+.
We had some very unusual weather here today. Normal Davis summers are very hot and dry and today should have been around 90-100F but it was rainy and cold! Global climate change perhaps?
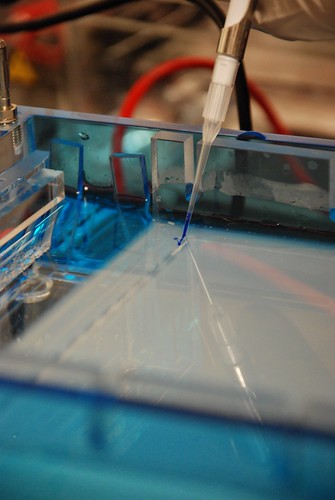
We realized today that we needed to test some carb plates that we made last week. Our suspicions arose when 16 ligations that had previously been transformed successfully failed to grow on our new plates. We did a quick test by streaking a couple plates with a part in an AK backbone. We streaked the same part on both Kan and Carb plates and only the Kan plates had any growth on them. We will make some new Carb plates tomorrow and hopefully they'll work! Today we also designed the rest of our Gibson assembly primers for constructing our screening plasmid. We'll order tomorrow and in about a week be able to assemble everything fairly quickly. In the meantime we'll continue using biobrick assembly.
We did PCR of E0240 with and without B0034. We wrote our formal letter to Novozymes and set up digest with E and P of E0240 modified with and without B0034. We also got a new strain of E. coli, BW22826 which is F-, Δ(araD-araB)567, Δ(codB-lacI)3, &lambda-, rpoS396(Am), rph-1. This strain will be helpful for us since it is both araBAD and LacI negative. This means we won't have to put an arabinose repressor in our plasmid which will cut down on construction time and allow for more testing time! We also needed the strain to be ∆LacI since we will be testing the Lac repressor and operator.
We also made carbenicillin plates and diagnosed the "bad" carb plates as chloramphenocol plates. Our mistake came when we used an older stock of "carb" which was labelled only with a 'c'. Lesson learned; don't assume anything!
Transformed all of our LacI mutants again along with E0240+psb1k3. All of the LacI mutants were plated on newly made Carb plates. Hopefully they have the correct antibiotic this time! We also got a new strain, JS006, which we requested from another lab. We'll make glycerol stocks and competent cells of these.

 "
"




