Team:Freiburg/Modelling
From 2011.igem.org
(Difference between revisions)
SophieCramer (Talk | contribs) (→imposing desired functions) |
SophieCramer (Talk | contribs) (→imposing desired functions) |
||
| (9 intermediate revisions not shown) | |||
| Line 17: | Line 17: | ||
The Construction of a new protein | The Construction of a new protein | ||
After a long and detailed search, we found out that it is most reasonable to use bacterial LRR motifs, since they seemed very well conserved in sequence, and they are the shortest – with only 21 amino acids per LRR repeat. (Wei 2008, Kajava 1998). We wanted to have a protein as simple and as predictable in behavior and structure as possible. | After a long and detailed search, we found out that it is most reasonable to use bacterial LRR motifs, since they seemed very well conserved in sequence, and they are the shortest – with only 21 amino acids per LRR repeat. (Wei 2008, Kajava 1998). We wanted to have a protein as simple and as predictable in behavior and structure as possible. | ||
| - | Several search inquiries led to the most conserved and shortest of all bacterial LRR (PDB: | + | Several search inquiries led to the most conserved and shortest of all bacterial LRR (PDB: 3CVR), unluckily this protein is a toxin derived from ''Yersinia pestis''. We of course did not want to mess around with toxins, |
{| style="color:black; background-color:lightgrey;" cellpadding="10%" cellpadding="15%" cellspacing="0" border="1" align=right | {| style="color:black; background-color:lightgrey;" cellpadding="10%" cellpadding="15%" cellspacing="0" border="1" align=right | ||
|[[File:Freiburg11_Seq1.png|250px]] | |[[File:Freiburg11_Seq1.png|250px]] | ||
| Line 44: | Line 44: | ||
===imposing desired functions=== | ===imposing desired functions=== | ||
The next consideration we had to do was: how many Nickel do we need on the surface of our ideal Nickel binding protein, in what pattern, with what distances between, and at which angles toward each other to allow proper ion complexion? | The next consideration we had to do was: how many Nickel do we need on the surface of our ideal Nickel binding protein, in what pattern, with what distances between, and at which angles toward each other to allow proper ion complexion? | ||
| - | Nickel can be complexed by imidazole structures from 4 planar orthogonal directions, as well as to axial positions. It can, however, only take four ligands at once - preferably in a planar orientation. Cobalt has a bipyramidal setup for ligand-binding, too, but can take up to six ligands. The distances from an N-atom in the Imidazole ring to the ion had to be between 3 and 6 Angström. We found a crystal structure of a different protein | + | Nickel can be complexed by imidazole structures from 4 planar orthogonal directions, as well as to axial positions. It can, however, only take four ligands at once - preferably in a planar orientation. Cobalt has a bipyramidal setup for ligand-binding, too, but can take up to six ligands. The distances from an N-atom in the Imidazole ring to the ion had to be between 3 and 6 Angström. We found a crystal structure of a different protein which was resolved with three Histidines complexing a Nickel ion for a comparison, as well as some old publications that analyzed peptide ion bonds that taught us how the complex should look like. (Jordan 1974) |
We wanted our protein to be short. First in order to cause as little unspecific binding as possible and further to allow an easy expression as well as for keeping costs low for gene synthesis. For a proper purification of His-tagged proteins in Ni-NTA columns we knew that there are up to three Nickel involved in the interaction between the His tag (which consists of 6 or 7 Histidines) and the column. These have to be in close proximity to one another. There are always two Histidines of the tag binding to one ion. | We wanted our protein to be short. First in order to cause as little unspecific binding as possible and further to allow an easy expression as well as for keeping costs low for gene synthesis. For a proper purification of His-tagged proteins in Ni-NTA columns we knew that there are up to three Nickel involved in the interaction between the His tag (which consists of 6 or 7 Histidines) and the column. These have to be in close proximity to one another. There are always two Histidines of the tag binding to one ion. | ||
| Line 59: | Line 59: | ||
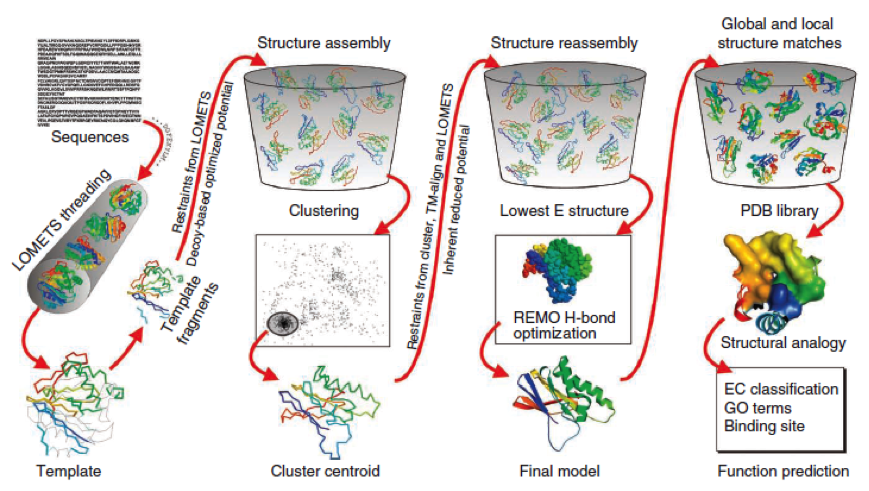
The community-wide Critical Assessment of Structure Prediction (CASP) ranked the I-Tasser as the best protein prediction method in the server section. | The community-wide Critical Assessment of Structure Prediction (CASP) ranked the I-Tasser as the best protein prediction method in the server section. | ||
The method of the I-Tasser can be divided into 4 stages: | The method of the I-Tasser can be divided into 4 stages: | ||
| - | Stage 1 is called threading. In this stage the sequence or parts of the sequence or motifs are compared with already known protein structures to identify evolutionary relatives and predict secondary structures. This gives a sequence profile and together with the query sequence it is threaded by LOMETS, a server combining seven threading programs which evaluate the sequence respectively the templates with an individual score | + | Stage 1 is called threading. In this stage the sequence or parts of the sequence or motifs are compared with already known protein structures to identify evolutionary relatives and predict secondary structures. This gives a sequence profile and together with the query sequence it is threaded by LOMETS, a server combining seven threading programs which evaluate the sequence respectively the templates with an individual score . The top templates are selected for further consideration. |
Stage 2 is responsible for structural assembly. The structure is modeled ab initio by aligning different templates from the threading. This is a very complex process and different programs are involved. | Stage 2 is responsible for structural assembly. The structure is modeled ab initio by aligning different templates from the threading. This is a very complex process and different programs are involved. | ||
Stage 3 is a model selection of the achievements of stage 2 with a following refinement of the predicted models. | Stage 3 is a model selection of the achievements of stage 2 with a following refinement of the predicted models. | ||
Stage 4 announces the function of the predicted molecule by its structural conformation comparing with already known proteins of PDB database. | Stage 4 announces the function of the predicted molecule by its structural conformation comparing with already known proteins of PDB database. | ||
| - | The predicted models are all evaluated by the C-Score, which assesses the quality of the prediction. It has a value from -5 to 2; the more positive the C-Score the merrier | + | The predicted models are all evaluated by the C-Score, which assesses the quality of the prediction. It has a value from -5 to 2; the more positive the C-Score the merrier and more plausible the predicted structure is. |
[[File:Freiburg11Modelling3.png|750px]] | [[File:Freiburg11Modelling3.png|750px]] | ||
| Line 76: | Line 76: | ||
{| style="color:black; background-color:lightgrey;" cellpadding="10%" cellpadding="15%" cellspacing="0" border="1" align="left" | {| style="color:black; background-color:lightgrey;" cellpadding="10%" cellpadding="15%" cellspacing="0" border="1" align="left" | ||
|[[File:Freiburg11_Seq7.png|300px]] | |[[File:Freiburg11_Seq7.png|300px]] | ||
| - | first | + | first aminoacid sequence submitted to I-TASSER to determine |
| + | |||
| + | proper Histidine patterns on the LRR backbone's surface | ||
|} | |} | ||
{| style="color:black; background-color:lightgrey;" cellpadding="10%" cellpadding="15%" cellspacing="0" border="1" align="right" | {| style="color:black; background-color:lightgrey;" cellpadding="10%" cellpadding="15%" cellspacing="0" border="1" align="right" | ||
|[[File:Freiburg11Modelling4.png|250px]] | |[[File:Freiburg11Modelling4.png|250px]] | ||
| - | + | first predicted structure used to determine proper Histidine | |
| + | |||
| + | patterns on the LRR backbone's surface | ||
|} | |} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| Line 88: | Line 101: | ||
{| style="color:black; background-color:lightgrey;" cellpadding="10%" cellpadding="15%" cellspacing="0" border="1" align="right" | {| style="color:black; background-color:lightgrey;" cellpadding="10%" cellpadding="15%" cellspacing="0" border="1" align="right" | ||
|[[File:Freiburg11Modelling5.png|250px]] | |[[File:Freiburg11Modelling5.png|250px]] | ||
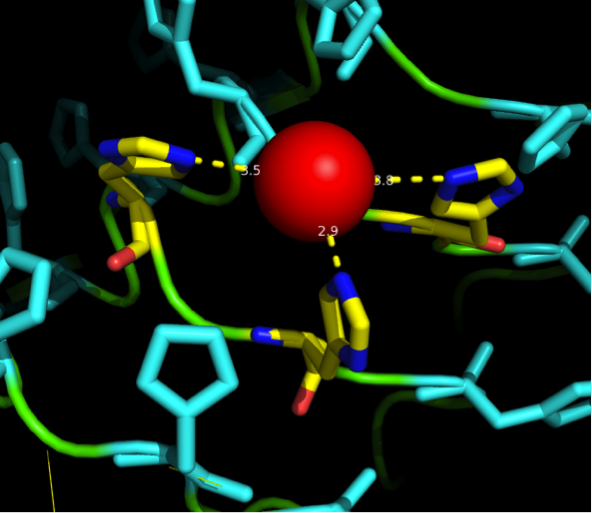
| - | + | superimposition of a Nickel ion into our first I-TASSER structure. | |
| + | |||
| + | Dotted lines show the distances between the atoms, angles were | ||
| + | |||
| + | analysed to see whether binding is likely to happen or not. | ||
| + | |||
| + | Histidine residues were analysed for flexibility in the backbone | ||
| + | |||
| + | and their potential to orient towards optimal binding. | ||
|} | |} | ||
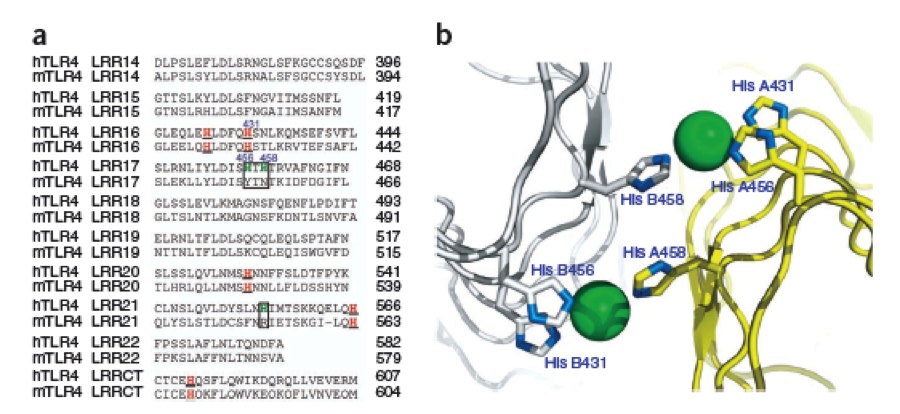
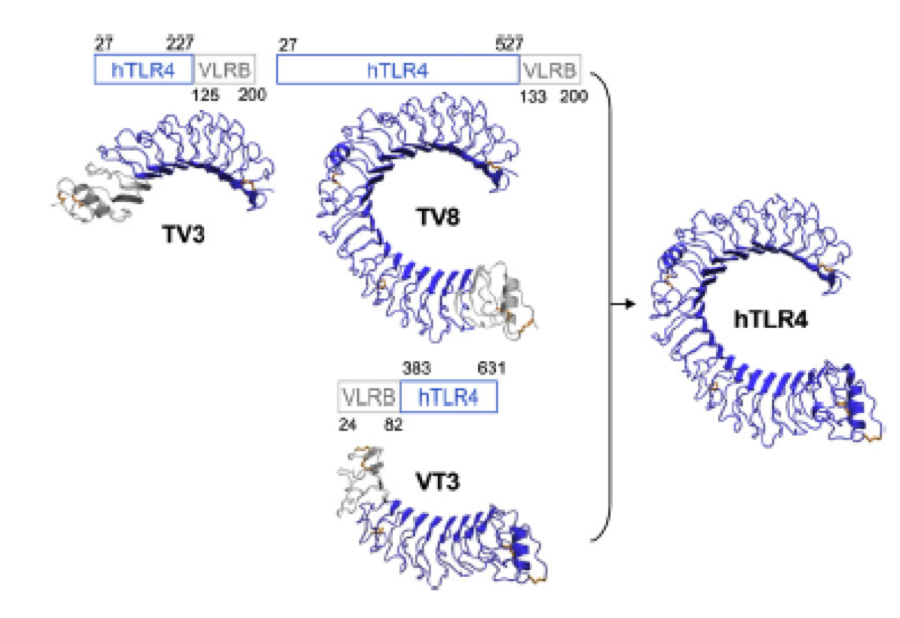
A solution to this was shown by Schmidt et. al. 2010, who crystallized the TLR-4 receptor. | A solution to this was shown by Schmidt et. al. 2010, who crystallized the TLR-4 receptor. | ||
| - | In this very useful work he dissected the TLR4(PDB: 3FXI) | + | In this very useful work he dissected the TLR4(PDB: 3FXI) into 3 parts, since it was too large and unhandy to be crystallized at once.. |
| Line 114: | Line 135: | ||
{| style="color:black; background-color:lightgrey;" cellpadding="10%" cellpadding="15%" cellspacing="0" border="1" align="right" | {| style="color:black; background-color:lightgrey;" cellpadding="10%" cellpadding="15%" cellspacing="0" border="1" align="right" | ||
|[[File:Freiburg11Modelling6.png|500px]] | |[[File:Freiburg11Modelling6.png|500px]] | ||
| - | + | Kim, Ho Min 2007: TLR-4 receptor in 3 pieces for crystallisation. Each piece was capped by Hagfish domains. Afterwards all three pieces were assembled by superimposition. | |
|} | |} | ||
| Line 127: | Line 148: | ||
| style="width: "30%";background-color:lightgrey;" | | | style="width: "30%";background-color:lightgrey;" | | ||
; [[File:Freiburg11_Seq4.png|200px]] | ; [[File:Freiburg11_Seq4.png|200px]] | ||
| - | |||
| style="width: "30%";background-color:lightgrey;" | | | style="width: "30%";background-color:lightgrey;" | | ||
; [[File:Freiburg11_Seq5.png|200px]] | ; [[File:Freiburg11_Seq5.png|200px]] | ||
| - | |||
|[[File:Freiburg11_Seq3.png|200px]] | |[[File:Freiburg11_Seq3.png|200px]] | ||
| - | |||
|} | |} | ||
| - | |||
===optimzing the sequence=== | ===optimzing the sequence=== | ||
| Line 141: | Line 158: | ||
| style="width: 100%;background-color:lightgrey;" | | | style="width: 100%;background-color:lightgrey;" | | ||
;[[File:Freiburg11_Seq6.png|700px]] | ;[[File:Freiburg11_Seq6.png|700px]] | ||
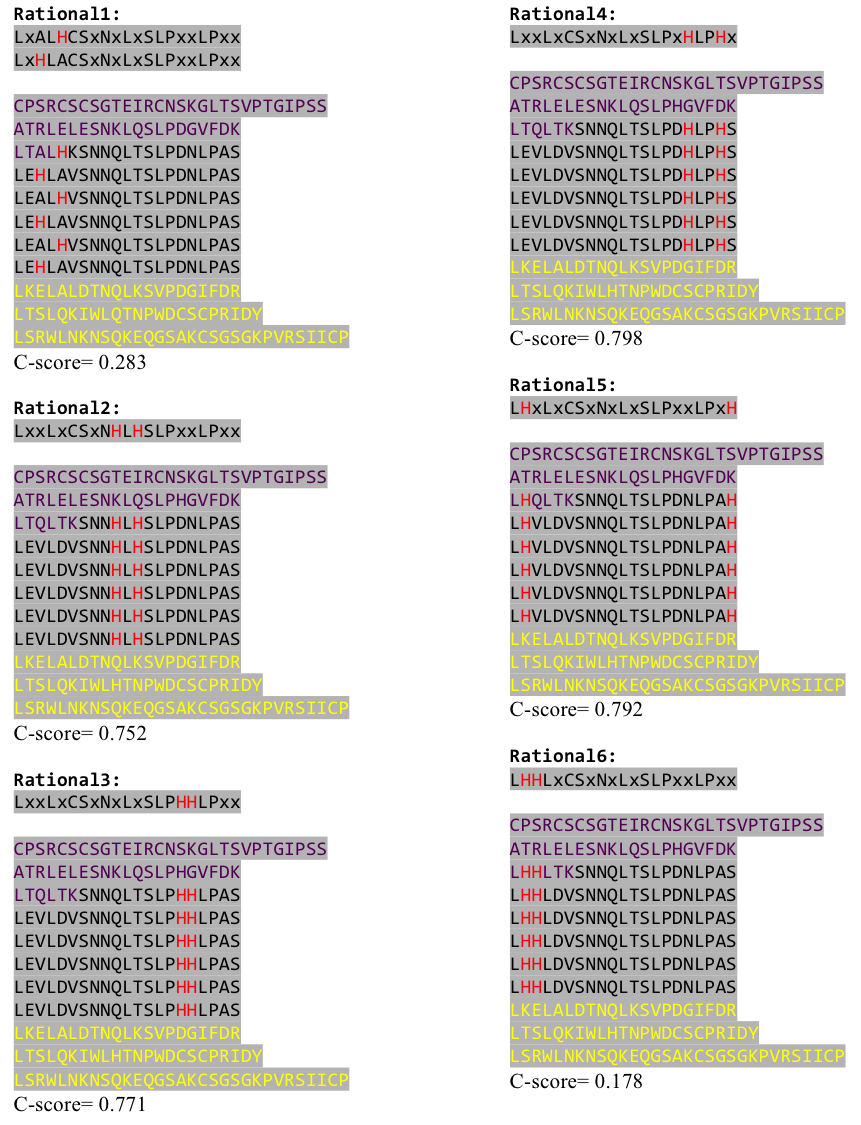
| - | + | Six different Histidine patterns inserted into our ideal LRR sequence, capped by Hagfish domain fragments were sent for I-TASSER structure prediction. C-Score shown under the sequence. | |
|} | |} | ||
Latest revision as of 03:54, 22 September 2011
 "
"
 Contact
Contact