Team:Debrecen Hungary/More
From 2011.igem.org
(→Arsenic) |
(→Hosting High School Students) |
||
| (32 intermediate revisions not shown) | |||
| Line 16: | Line 16: | ||
All of our work complies with the University of Debrecen's local biosafety and bioethics regulations. Our project leader and team instructors oversaw the biological safety issue throughout the whole working time this year. There is also a biosafety supervisor who supervises the overall laboratory work biosecurity-wise. All team members who worked at the bench this summer have had proper safety training. We received approval from all overseeing groups. | All of our work complies with the University of Debrecen's local biosafety and bioethics regulations. Our project leader and team instructors oversaw the biological safety issue throughout the whole working time this year. There is also a biosafety supervisor who supervises the overall laboratory work biosecurity-wise. All team members who worked at the bench this summer have had proper safety training. We received approval from all overseeing groups. | ||
| - | + | '''<font color="green">4. Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?</font>''' | |
| - | + | ||
| - | + | An important tool to raise interest and knowledge in safety issues is education. Open source webpages containing regulations, new ideas, video tutorials etc. can be designed. Online forums, trainings, courses should be organized in order to share new issues between groups and experts. | |
| + | =='''Testing of arsenic biosensor'''== | ||
| - | ... | + | ==='''Background'''=== |
| + | |||
| + | Arsenic is a serious groundwater contaminant of major public health significance. During the 1970s, due to a high incidence of waterborne disease, millions of tube wells were drilled in Bangladesh to provide drinking water. Unfortunately, years later, many instances of ill health were found to be associated with consumption of this water, and it became apparent that many of the wells had inadvertently been drilled through arsenic bearing sediments and that the water was contaminated with arsenic in the form of arsenate and arsenite anions. Arsenic is a serious poison, and chronic consumption leads to arsenicosis, with symptoms such as skin lesions and cancers. The WHO limit for arsenic in drinking water is 10 ppb, though a more relaxed limit of 50 ppb is still in operation in many countries. Many wells exceed these limits. Similar contaminated groundwater has also been found to occur in many other countries, and one estimate suggests that 100 million people worldwide may be at risk. There is therefore a need for methods for testing and monitoring arsenic levels. Various highly accurate laboratory methods are available, but for large scale use in less developed regions, it would be preferable to have simple and cheap test kits which can be used in the field. | ||
| + | |||
| + | In 2006, Team Edinburgh have developed a genetically engineered machine which was able to detect arsenite/arsenate ions in water samples. They particitaped first time and won first prize in the Best Real World Application category.To see the part (BBa_33203) visit the Registry of Standard Biological Parts here: [http://partsregistry.org/Part:BBa_J33203]. | ||
| + | |||
| + | As the South-East of Hungary is highly affected by the arsenic problem (there are wells in which the level of arsenic exceeds the allowed limit), we had the possibility to use real-world samples for testing. The current situation of the arseinc problem in the drinking water of the south east region of Hungary can be found here: [https://springerlink3.metapress.com/content/q666u6412x62276l/resource-secured/?target=fulltext.pdf&sid=jozwwr1csp0dyx2bvk1iztke&sh=www.springerlink.com] | ||
| + | |||
| + | As the Edinburgh team tested the system only on distilled water samples spiked with sodium-arsenate, we contacted the team and offered our help to test the machine on real-world samples to prove that it works not only on artificial but on natural samples as well (with many different compounds that may interfere with the part's action). | ||
| + | We also sent them twenty sterilized samples collected by a Local Public Health Organisation from different wells - this organisation has also provided data on arsenic concentrations gained from flame atomic absorption spectrometry measurements. | ||
| + | |||
| + | === Method of testing === | ||
| + | |||
| + | The contact person of Team Edinburgh 2006 sent us the protocol should be used according to their experiments. | ||
| + | We collected samples from two wells which were known to contain elevated arsenic concentration (Szegfű and Madách street, Békéscsaba, Hungary). Find these places using Google Maps: [http://maps.google.com/maps?f=q&source=s_q&hl=hu&q=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&sll=37.09024,-96.503906&sspn=34.945679,92.285156&ie=UTF8&cd=1&geocode=FeAfyAIdRVlBAQ&split=0&hq=&hnear=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&ll=46.666579,21.059632&spn=0.021204,0.044546&t=h&z=15], [http://maps.google.com/maps?f=q&source=s_q&hl=hu&geocode=&q=5600+B%C3%A9k%C3%A9scsaba,+Mad%C3%A1ch+utca,+Magyarorsz%C3%A1g&sll=46.666579,21.059632&sspn=0.021204,0.044546&g=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&ie=UTF8&hq=&hnear=5600+B%C3%A9k%C3%A9scsaba,+Mad%C3%A1ch+utca,+Magyarorsz%C3%A1g&ll=46.666314,21.070104&spn=0.021204,0.044546&t=h&z=15] | ||
| + | |||
| + | We used DH5 alpha E. coli cell line with lacZDM15 mutation as host cells, than we prepared a medium consisting of: | ||
| + | * 2.0 g/l peptone | ||
| + | * 0.1 g/l yeast extract | ||
| + | * 0.3 g/l K2HPO4 | ||
| + | * 2.1 g/l NaHCO3 | ||
| + | * a pinch of the indiciator powder: Bromothymol blue | ||
| + | * 10 g/l lactose | ||
| + | * ''Autoclaved or filter-sterilized water sample dilutions'' | ||
| + | |||
| + | We spiked these media with transformed bacterial cells containing the arsenic biosensor system, and incubated 24 hours at 37°C. | ||
| + | |||
| + | === Results === | ||
| + | |||
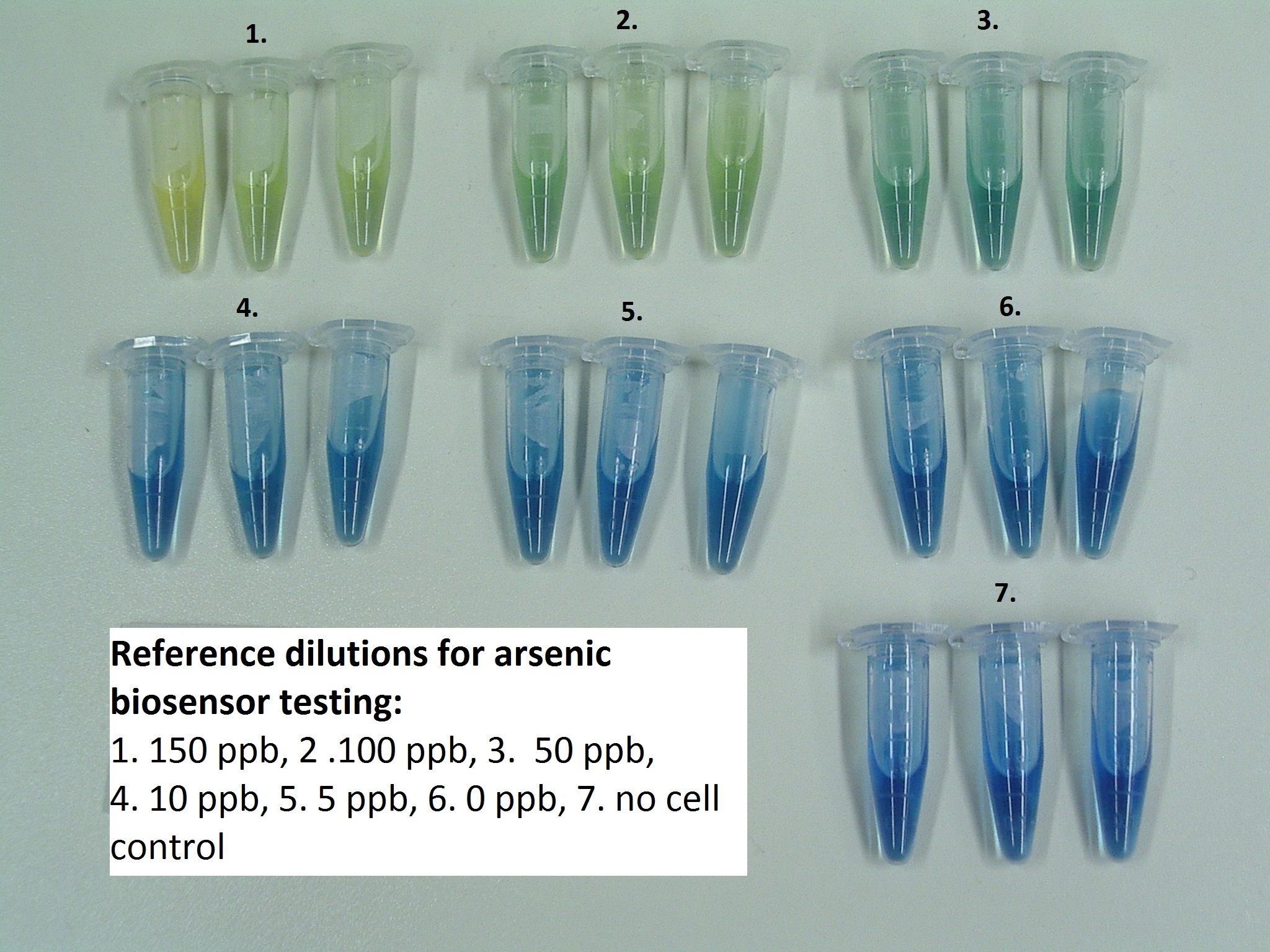
| + | We received that the part works also on real world samples, not only on samples made of water and sodium-arsenate. The colour change of Bromothymol Blue seemed to be directly proportional to the concentration of arsenic. According to the reference dilutions made from Na-arsenate and MilliQ water the concentration of arsenic in our collected samples is at least 15-fold higher than the WHO recommended limit (Fig.1., Fig.2., Fig.3.). | ||
| + | |||
| + | [[File:arzén.jpg|400px|center|thumb|Fig.1.: Arsenic reference dilutions for estimating arsenic concentration of real world samples after 24 hours of incubation.]] | ||
| + | |||
| + | [[File:arzén1.jpg|400px|center|thumb|Fig.2.: Dilutions of Szegfű street sample after 24 hours of incubation.]] | ||
| + | |||
| + | [[File:arzén2.jpg|400px|center|thumb|Fig.3.: Dilutions of Madách street sample after 24 hours of incubation.]] | ||
| + | |||
| + | As the result of the successful cooperation, an article was published on the topic: | ||
| + | |||
| + | K. de Mora, N. Joshi, B. L. Balint et al. "A pH-based biosensor for detection of arsenic in drinking water" Anal Bioanal Chem. 2011 May;400(4):1031-9. Epub 2011 Mar 27. | ||
| + | |||
| + | Link: [http://www.ncbi.nlm.nih.gov/pubmed?term=balint%20arsenic] | ||
| + | |||
| + | =='''Hosting High School Students'''== | ||
| + | |||
| + | Having seen the difference between a first-year and a second-year iGEM team, we felt exrtremely important to recruit new members to the group, to keep the project going. One side of this task was accomlished within the walls of our university by holding lectures and presentations about the iGEM-experience for students and inviting them to visit the lab and participate in the competition. | ||
| + | |||
| + | We also decided to recruit students at an even earlier peroid of their studies, so a lecture was put together and held at a local secondary school. During this presentation the basics of synthetic biology, the main features of the iGEM competition and some important results from our and some other groups were discussed. Students with a special interest in biology were at this meeting. Some of them took the opportunity and visited the group at the lab and one of them decided to join us and helped our work this year. | ||
| + | |||
| + | This year we got to know a smart, anthusiastic, helpful High School student called Éva, who became an indispensable member of the Team. She came not for only few weeks but for the whole summer, and gained experience which maybe pushes her toward biology studies. | ||
| + | |||
| + | [[File:Evi hosting.jpg|center|300px|thumb|Eva is preparing gel for agarose gel electrophoresis.]] | ||
Latest revision as of 21:43, 21 September 2011
Safety
1. Would any of your project ideas raise safety issues in terms of researcher safety, public safety, or environmental safety?
No issue of researcher safety, public safety or environmental safety were raised during Debrecen's iGEM 2011 project. We only worked with non-hazardous, non-infectious, commonly used and accepted bacteria strain (DH5α) and mammalian cancer cell lines (COS-1). When working with toxic chemicals (e.g. ethidiumbromide or estrogen), nitrile gloves, and white coats were worn. All of the work was conducted in a biosafety level S1 laboratory. Rules of the best microbiological practices were applied.
2. Do any of the new BioBrick parts (or devices) that you made this year raise any safety issues?
All material handled or distributed are non-hazardous and non-infectious. It agrees with all safety standards requested biosafety level 1, therfore the project get a full supports for the work done by the iGEM team. We developed synthetic LBD's for use in mammalian cells in fusion parts. These parts are completely harmless, no matter what organism they are transformed / transfected into.
3. Is there a local biosafety group, committee, or review board at your institution?
All of our work complies with the University of Debrecen's local biosafety and bioethics regulations. Our project leader and team instructors oversaw the biological safety issue throughout the whole working time this year. There is also a biosafety supervisor who supervises the overall laboratory work biosecurity-wise. All team members who worked at the bench this summer have had proper safety training. We received approval from all overseeing groups.
4. Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?
An important tool to raise interest and knowledge in safety issues is education. Open source webpages containing regulations, new ideas, video tutorials etc. can be designed. Online forums, trainings, courses should be organized in order to share new issues between groups and experts.
Testing of arsenic biosensor
Background
Arsenic is a serious groundwater contaminant of major public health significance. During the 1970s, due to a high incidence of waterborne disease, millions of tube wells were drilled in Bangladesh to provide drinking water. Unfortunately, years later, many instances of ill health were found to be associated with consumption of this water, and it became apparent that many of the wells had inadvertently been drilled through arsenic bearing sediments and that the water was contaminated with arsenic in the form of arsenate and arsenite anions. Arsenic is a serious poison, and chronic consumption leads to arsenicosis, with symptoms such as skin lesions and cancers. The WHO limit for arsenic in drinking water is 10 ppb, though a more relaxed limit of 50 ppb is still in operation in many countries. Many wells exceed these limits. Similar contaminated groundwater has also been found to occur in many other countries, and one estimate suggests that 100 million people worldwide may be at risk. There is therefore a need for methods for testing and monitoring arsenic levels. Various highly accurate laboratory methods are available, but for large scale use in less developed regions, it would be preferable to have simple and cheap test kits which can be used in the field.
In 2006, Team Edinburgh have developed a genetically engineered machine which was able to detect arsenite/arsenate ions in water samples. They particitaped first time and won first prize in the Best Real World Application category.To see the part (BBa_33203) visit the Registry of Standard Biological Parts here: [http://partsregistry.org/Part:BBa_J33203].
As the South-East of Hungary is highly affected by the arsenic problem (there are wells in which the level of arsenic exceeds the allowed limit), we had the possibility to use real-world samples for testing. The current situation of the arseinc problem in the drinking water of the south east region of Hungary can be found here: [1]
As the Edinburgh team tested the system only on distilled water samples spiked with sodium-arsenate, we contacted the team and offered our help to test the machine on real-world samples to prove that it works not only on artificial but on natural samples as well (with many different compounds that may interfere with the part's action). We also sent them twenty sterilized samples collected by a Local Public Health Organisation from different wells - this organisation has also provided data on arsenic concentrations gained from flame atomic absorption spectrometry measurements.
Method of testing
The contact person of Team Edinburgh 2006 sent us the protocol should be used according to their experiments. We collected samples from two wells which were known to contain elevated arsenic concentration (Szegfű and Madách street, Békéscsaba, Hungary). Find these places using Google Maps: [http://maps.google.com/maps?f=q&source=s_q&hl=hu&q=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&sll=37.09024,-96.503906&sspn=34.945679,92.285156&ie=UTF8&cd=1&geocode=FeAfyAIdRVlBAQ&split=0&hq=&hnear=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&ll=46.666579,21.059632&spn=0.021204,0.044546&t=h&z=15], [http://maps.google.com/maps?f=q&source=s_q&hl=hu&geocode=&q=5600+B%C3%A9k%C3%A9scsaba,+Mad%C3%A1ch+utca,+Magyarorsz%C3%A1g&sll=46.666579,21.059632&sspn=0.021204,0.044546&g=5600+B%C3%A9k%C3%A9scsaba,+Szegf%C5%B1+utca,+Magyarorsz%C3%A1g&ie=UTF8&hq=&hnear=5600+B%C3%A9k%C3%A9scsaba,+Mad%C3%A1ch+utca,+Magyarorsz%C3%A1g&ll=46.666314,21.070104&spn=0.021204,0.044546&t=h&z=15]
We used DH5 alpha E. coli cell line with lacZDM15 mutation as host cells, than we prepared a medium consisting of:
- 2.0 g/l peptone
- 0.1 g/l yeast extract
- 0.3 g/l K2HPO4
- 2.1 g/l NaHCO3
- a pinch of the indiciator powder: Bromothymol blue
- 10 g/l lactose
- Autoclaved or filter-sterilized water sample dilutions
We spiked these media with transformed bacterial cells containing the arsenic biosensor system, and incubated 24 hours at 37°C.
Results
We received that the part works also on real world samples, not only on samples made of water and sodium-arsenate. The colour change of Bromothymol Blue seemed to be directly proportional to the concentration of arsenic. According to the reference dilutions made from Na-arsenate and MilliQ water the concentration of arsenic in our collected samples is at least 15-fold higher than the WHO recommended limit (Fig.1., Fig.2., Fig.3.).
As the result of the successful cooperation, an article was published on the topic:
K. de Mora, N. Joshi, B. L. Balint et al. "A pH-based biosensor for detection of arsenic in drinking water" Anal Bioanal Chem. 2011 May;400(4):1031-9. Epub 2011 Mar 27.
Link: [http://www.ncbi.nlm.nih.gov/pubmed?term=balint%20arsenic]
Hosting High School Students
Having seen the difference between a first-year and a second-year iGEM team, we felt exrtremely important to recruit new members to the group, to keep the project going. One side of this task was accomlished within the walls of our university by holding lectures and presentations about the iGEM-experience for students and inviting them to visit the lab and participate in the competition.
We also decided to recruit students at an even earlier peroid of their studies, so a lecture was put together and held at a local secondary school. During this presentation the basics of synthetic biology, the main features of the iGEM competition and some important results from our and some other groups were discussed. Students with a special interest in biology were at this meeting. Some of them took the opportunity and visited the group at the lab and one of them decided to join us and helped our work this year.
This year we got to know a smart, anthusiastic, helpful High School student called Éva, who became an indispensable member of the Team. She came not for only few weeks but for the whole summer, and gained experience which maybe pushes her toward biology studies.
 "
"