Team:Glasgow/Parts
From 2011.igem.org
Chris Wood (Talk | contribs) |
Chris Wood (Talk | contribs) |
||
| Line 29: | Line 29: | ||
"A blue light-inducible phosphodiesterase activity in the cyanobacterium <i>Synechococcus elongates</i>" which can be accessed <a href=http://www.ncbi.nlm.nih.gov/pubmed/20408974>here.</a> | "A blue light-inducible phosphodiesterase activity in the cyanobacterium <i>Synechococcus elongates</i>" which can be accessed <a href=http://www.ncbi.nlm.nih.gov/pubmed/20408974>here.</a> | ||
| - | <h1>Multiple Cloning Site (MCS) Biobrick<h1> | + | <h1>Multiple Cloning Site (MCS) Biobrick</h1> |
<p>We wanted to test multiple genes with the same promoter, RBS and terminator to characterise PDE, latherin, latherin with HIS tag, ranaspumin and ranaspumin with HIS tag , which are some of our novel biobricks. To ligate one of these constructs together requires three or four (three if you’re using the 3A method) restriction and ligation reactions. So to test all five under the same promoter, RBS and terminator would require at the very least 15 restriction and ligation reactions. However using our multiple cloning site (MCS) biobrick it is possible to create the promoter, RBS, MCS and terminator construct in three-four ligations before inserting each gene into the MCS with just one extra restriction and ligation step. So it would be possible to have all five constructs made in just nine restriction and ligation reactions, saving a huge amount of time. And for each extra gene you want to test this would only require one extra restriction and ligation rather than three or four. This is great for characterisation of a large number of biobricks. We suggest that if you plan to use the MCS biobrick, when you are adding biobrick ends to your PCR primers or sequence for synthesis , add one of the restriction sites included in our MCS before and after the whole construct. As long as you ensure the gene is still in-frame then you can easily insert it into your standard construct for testing. When it comes to submission, just restrict the novel biobrick out of the characterisation construct and into ligate your submission vector . Restricting with X+S only just your gene will drop out. Restricting with E+P will give fragments for your promoter+ RBS and terminator as well as your gene however the gene will usually be much larger than the fragments allowing for gel purification of only the restricted gene.<p> | <p>We wanted to test multiple genes with the same promoter, RBS and terminator to characterise PDE, latherin, latherin with HIS tag, ranaspumin and ranaspumin with HIS tag , which are some of our novel biobricks. To ligate one of these constructs together requires three or four (three if you’re using the 3A method) restriction and ligation reactions. So to test all five under the same promoter, RBS and terminator would require at the very least 15 restriction and ligation reactions. However using our multiple cloning site (MCS) biobrick it is possible to create the promoter, RBS, MCS and terminator construct in three-four ligations before inserting each gene into the MCS with just one extra restriction and ligation step. So it would be possible to have all five constructs made in just nine restriction and ligation reactions, saving a huge amount of time. And for each extra gene you want to test this would only require one extra restriction and ligation rather than three or four. This is great for characterisation of a large number of biobricks. We suggest that if you plan to use the MCS biobrick, when you are adding biobrick ends to your PCR primers or sequence for synthesis , add one of the restriction sites included in our MCS before and after the whole construct. As long as you ensure the gene is still in-frame then you can easily insert it into your standard construct for testing. When it comes to submission, just restrict the novel biobrick out of the characterisation construct and into ligate your submission vector . Restricting with X+S only just your gene will drop out. Restricting with E+P will give fragments for your promoter+ RBS and terminator as well as your gene however the gene will usually be much larger than the fragments allowing for gel purification of only the restricted gene.<p> | ||
Revision as of 09:03, 20 September 2011

Parts
The 2011 University of Glasgow team has submitted 9 novel biobricks. You can find out more about these below, or by visiting the Registry Page. If you are interested in finding out about the safety reviews of these biobricks then please visit our Biobrick Safety Page, which can be accessed here.
Phosphodiesterase
Phosphodiesterase (Part BBa_K660300)Phosphodiesterases are a family of enzymes naturally present in microorganisms, which break phosphodiester bonds. The specific enzyme we are using breaks the phosphodiester bond in the second messenger nucleotide, cyclic digaunylate (cyclic-di-GMP). Cyclic-di-GMP is important in many bacterial processes, including biofilm formation and motility. The enzyme regulates signal transduction by controlling levels of the signalling molecule in cells.
This enzyme was amplified from the genome of Pseudomonas aeruginosa PA01. The enzyme contains a domain (vieA) which specifically targets the cyclic-di-GMP molecule. We are using the enzyme to control the levels of cyclic-di-GMP within the cell. We expect that the targeted expression of phosphodesiterase could be used to interfere with biofilm formation or to trigger dispersal.
Due to the number of processes in which cyclic-di-GMP is used by prokarytes, we hope that future iGEM teams will find diverse uses for it.
Phosphodiesterase with 6xHIS Tag (Part BBa_K660301)
We have also created a version of the phosphodiesterase biobrick with a 6xHIS tag. This can be used for affinity purification.
References & Further Reading:
"The EAL Domain Protein VieA Is a Cyclic Diguanylate Phosphodiesterase" which can be accessed here here.
"A blue light-inducible phosphodiesterase activity in the cyanobacterium Synechococcus elongates" which can be accessed here.
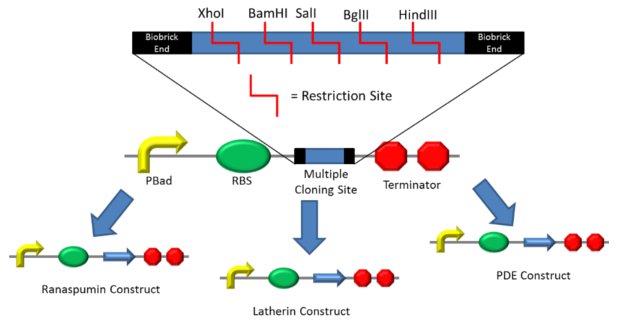
Multiple Cloning Site (MCS) Biobrick
We wanted to test multiple genes with the same promoter, RBS and terminator to characterise PDE, latherin, latherin with HIS tag, ranaspumin and ranaspumin with HIS tag , which are some of our novel biobricks. To ligate one of these constructs together requires three or four (three if you’re using the 3A method) restriction and ligation reactions. So to test all five under the same promoter, RBS and terminator would require at the very least 15 restriction and ligation reactions. However using our multiple cloning site (MCS) biobrick it is possible to create the promoter, RBS, MCS and terminator construct in three-four ligations before inserting each gene into the MCS with just one extra restriction and ligation step. So it would be possible to have all five constructs made in just nine restriction and ligation reactions, saving a huge amount of time. And for each extra gene you want to test this would only require one extra restriction and ligation rather than three or four. This is great for characterisation of a large number of biobricks. We suggest that if you plan to use the MCS biobrick, when you are adding biobrick ends to your PCR primers or sequence for synthesis , add one of the restriction sites included in our MCS before and after the whole construct. As long as you ensure the gene is still in-frame then you can easily insert it into your standard construct for testing. When it comes to submission, just restrict the novel biobrick out of the characterisation construct and into ligate your submission vector . Restricting with X+S only just your gene will drop out. Restricting with E+P will give fragments for your promoter+ RBS and terminator as well as your gene however the gene will usually be much larger than the fragments allowing for gel purification of only the restricted gene.
Novel Reporters
We have submitted two novel reporters to the registry, LOV2 and iLOV. Read on to find out about the exciting advantages these reporters have over GFP derived flourescent proteins.LOV2 (Part:BBa_K660000)
The LOV (Light-Oxygen-Voltage) domain is a photoreceptor that responds to blue light. In nature it was first found to be involved in the phototropism response in plants and has since been found to be present in fungi and bacteria also. It has been shown to be coupled to many domains, for example phosphodiesterase or kinases.
We are using it as a reporter due to its ability to function in anoxic conditions. This is particularly useful in biofilms and is a function that fluorescent proteins derived from GFP do not have.
iLOV (Part:BBa_K660004)
iLOV is a version of LOV2 that has been altered through site directed mutagenesis and DNA shuffling. iLOV has the same function and uses as LOV2, but contains mutations that caused increased intensity in brightness.
As a reporter, it is advantageous over GFP derived fluorescent proteins for a number of reasons. Firstly, it is small in size so can be used when tagging proteins. It also recovers quickly from photobleaching. Uniquely, it has the ability to function in anoxic conditions.
References & Further Reading:
"LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): Binding sites for the chromophore flavin mononucleotide" which can be accessed here.
"The LOV Domain Family: Photoresponsive Signaling Modules Coupled to Diverse Output Domains" which can be accessed here.
"Information on the LOV Domain" by Dr John Christie which can be accessed here.
"The photoreversible fluorescent protein iLOV outperforms GFP as a reporter of plant virus infection" which can be accessed here.
Surfactant Proteins
We have developed biobricks of surfactant proteins, Ranaspumin and Latherin, which have been investigated for their antimicrobial activity.Ranaspumin (RSN2) (Part:BBa_K660100)
Ranaspumin is a protein found in the foam nests of the Túngara Frog (Engystomops pustulosus). In nature it is used to protect and incubate the fertilized eggs of the frog.
It has natural antimicrobial and antibiofilm activity.
Ranaspumin has a unique amino acid sequence.
Ranspumin (RSN2) with 6xHIS tag (Part:BBa_K660101)
We have also created a version of Ranaspumin (RSN2) biobrick with a 6xHIS tag. This can be used for affinity purification.
References & Further Reading:
"Ranaspumin-2: Structure and Function of a Surfactant Protein from the Foam Nests of a Tropical Frog" which can be accessed here.
"Biofoams and natural protein surfactants" which can be accessed here.
Latherin (Part:BBa_K660200)
Latherin is a surfactant protein that was originally isolated from horse sweat. Its normal biological function is temperature regulation and is believed to function by enhancing evaporation from the pelt. Due to its ability to bind to hydrophobic surfaces, the protein is being investigated for its possible ability to aid in breaking up biofilms.
We are using latherin to aid in dispersal of biofilms, which is a natural property of the protein.
Latherin with 6xHIS tag (Part:BBa_K660201)
We have also created a version of Latherin with a 6xHIS tag. This can be used for affinity purification.
References & Further Reading:
"Latherin: A Surfactant Protein of Horse Sweat and Saliva" which can be accessed here
"Isolation and characterization of latherin, a surface-active protein from horse sweat" which can be accessed here.
"Biochemical characterization and surfactant properties of horse allergens" which can be accessed here.
"Latherin and other biocompatible surfactant proteins" which can be accessed here
 "
"