Team:Caltech/Week 9
From 2011.igem.org
| (5 intermediate revisions not shown) | |||
| Line 120: | Line 120: | ||
</table> | </table> | ||
==August 12== | ==August 12== | ||
| - | Continue time-dependent biofilm optimization experiment with crystal violet stain. | + | Continue time-dependent biofilm optimization experiment with crystal violet stain.<br/> |
| + | PCR of 16s. | ||
===Results=== | ===Results=== | ||
Biobrick transformation plates | Biobrick transformation plates | ||
| Line 141: | Line 142: | ||
</tr> | </tr> | ||
</table> | </table> | ||
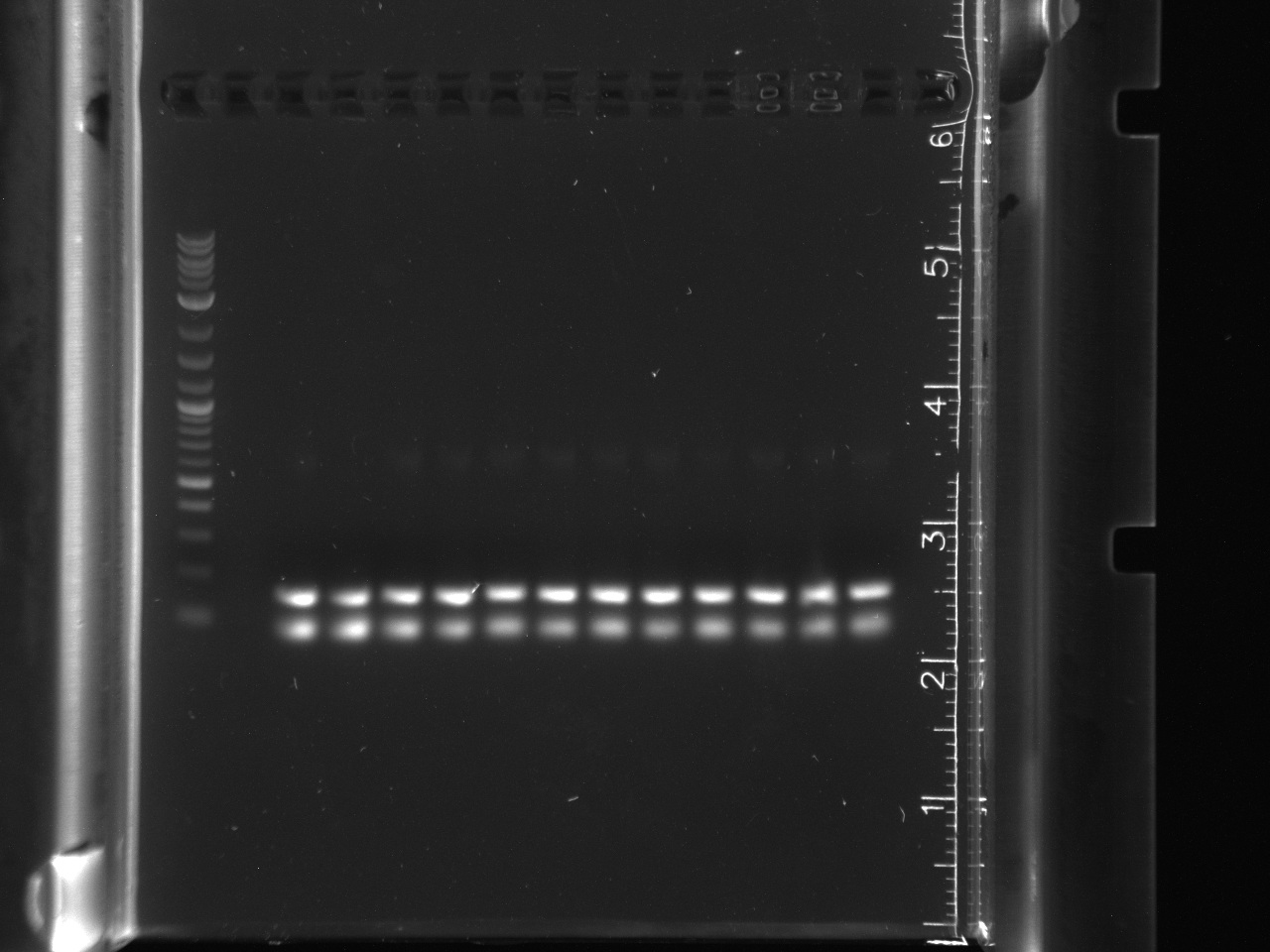
| + | [[File:8-1216sphusion.jpg|thumb|16s amplification, lane 1 NEB 2-log ladder, lane 2 9-3A, lane 3 9-3B, lane 4 9-3C, lane 5 9-9A, lane 6 9-9B, lane 7 9-9C, lane 8 10-3A, lane 9 10-3B, lane 10 10-3C, lane 11 10-6A, lane 12 10-6B, lane 13 10-6C, lane 14 Control 1, lane 15 Control 2]] | ||
==August 13== | ==August 13== | ||
===Results=== | ===Results=== | ||
| Line 226: | Line 228: | ||
<td>.881</td> | <td>.881</td> | ||
</tr> | </tr> | ||
| - | </table> | + | </table><br/> |
| + | <gallery> | ||
| + | File:Biofilm_graphs.jpg|Biofilm experiment chart | ||
| + | File:96-well.jpg|Biofilm experiment 96-well plate | ||
| + | File:Cuvettes.jpg|Biofilm experiment cuvettes | ||
| + | </gallery> | ||
}} | }} | ||
Latest revision as of 18:50, 8 September 2011
|
Project |
August 8Testing for biofilms grown on 96-well plate and Erlenmeyer flasks with crystal violet ResultsBacteria-incubated well remained cystal violet stained after several rinses, indicating the presence of a biofilm, in contrast to the clear control well. Got successful amplification of 16s in LA River sample 9-9. In both gels, the controls had no bands and all other sample lanes had no bands. August 9Evaporate-centrifuge BPA sample to concentrate for GCMS ResultsI732019 and I732005 plates had no colonies. August 10Minimal media transfers ResultsI732019 and I732005 plates had no colonies August 11Colony PCR of DDT gene colonies Results
August 12Continue time-dependent biofilm optimization experiment with crystal violet stain. ResultsBiobrick transformation plates
August 13Results
Crystal violet biofilm results:
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"