Team:XMU-China/Project/Description
From 2011.igem.org
(Difference between revisions)
(→Project Description) |
|||
| Line 16: | Line 16: | ||
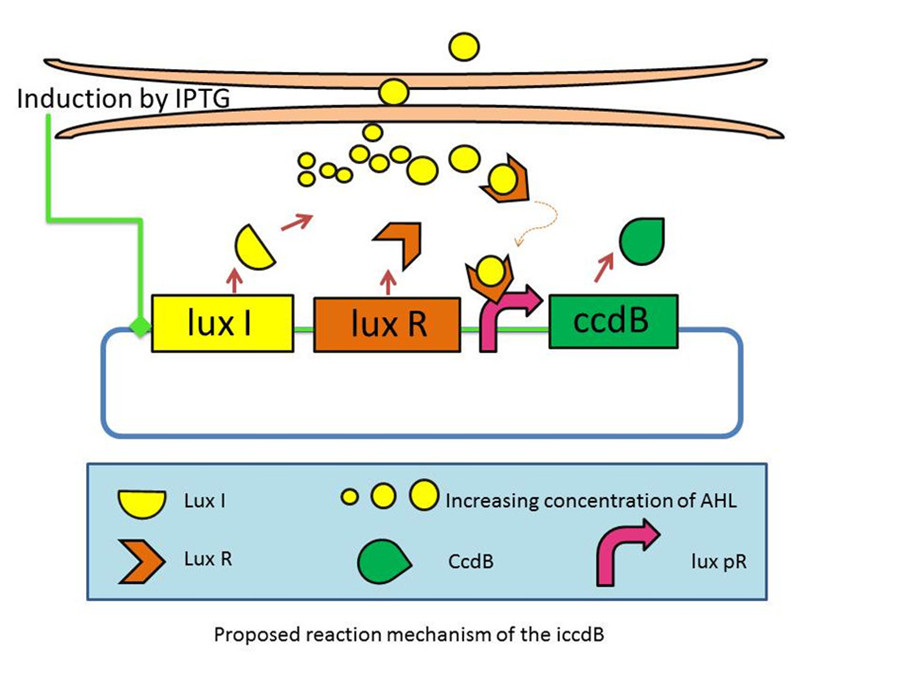
This circuit incorporates a mechanism for programmed death in respond to changes in the environment, and allows us to probe the design principles of its more complex natural counterparts. | This circuit incorporates a mechanism for programmed death in respond to changes in the environment, and allows us to probe the design principles of its more complex natural counterparts. | ||
| + | |||
| + | ==reference== | ||
| + | [1] Kempner E, Hanson F. Aspects of light production by Photobacterium fischeri[J]. Journal of Bacteriology, 1968, 95(3): 975-979. | ||
| + | [2] Fuqua WC, Winan SC, Greenberg E. Quorum sensing in bacteria: the luxR-luxI family of cell density-responsive transcriptional regulators[J]. Journal of Bacteriology, 1994, 176(2): 269-275. | ||
| + | [3] Baldwin T, Devine JH, Heckel, RC, Lin, JW, Shadel GS. The complete nucleotide sequence of the lux regulon of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence[J]. Journal of Bioluminescence and Chemiluminescence, 1989, 4(1): 326-341. | ||
| + | [4] You L, Cox RS, Weiss R, Arnold FH. Programmed population control by cell-cell communication and regulated killing[J]. Nature, 2004, 428(6985): 868-871. | ||
| + | [5] Kampranis SC, Howells AJ, Maxwell A. The interaction of DNA gyrase with the bacterialtoxin CcdB: evidence for the existence of two gyrase-CcdB complexes[J]. Journal of Molecular Biology, 1999, 293(3): 733-744. | ||
Revision as of 12:40, 28 October 2011
 "
"