Team:Washington/Celiacs/Background
From 2011.igem.org
(→Enzyme Identification) |
(→Introduction) |
||
| Line 5: | Line 5: | ||
====Introduction==== | ====Introduction==== | ||
| + | |||
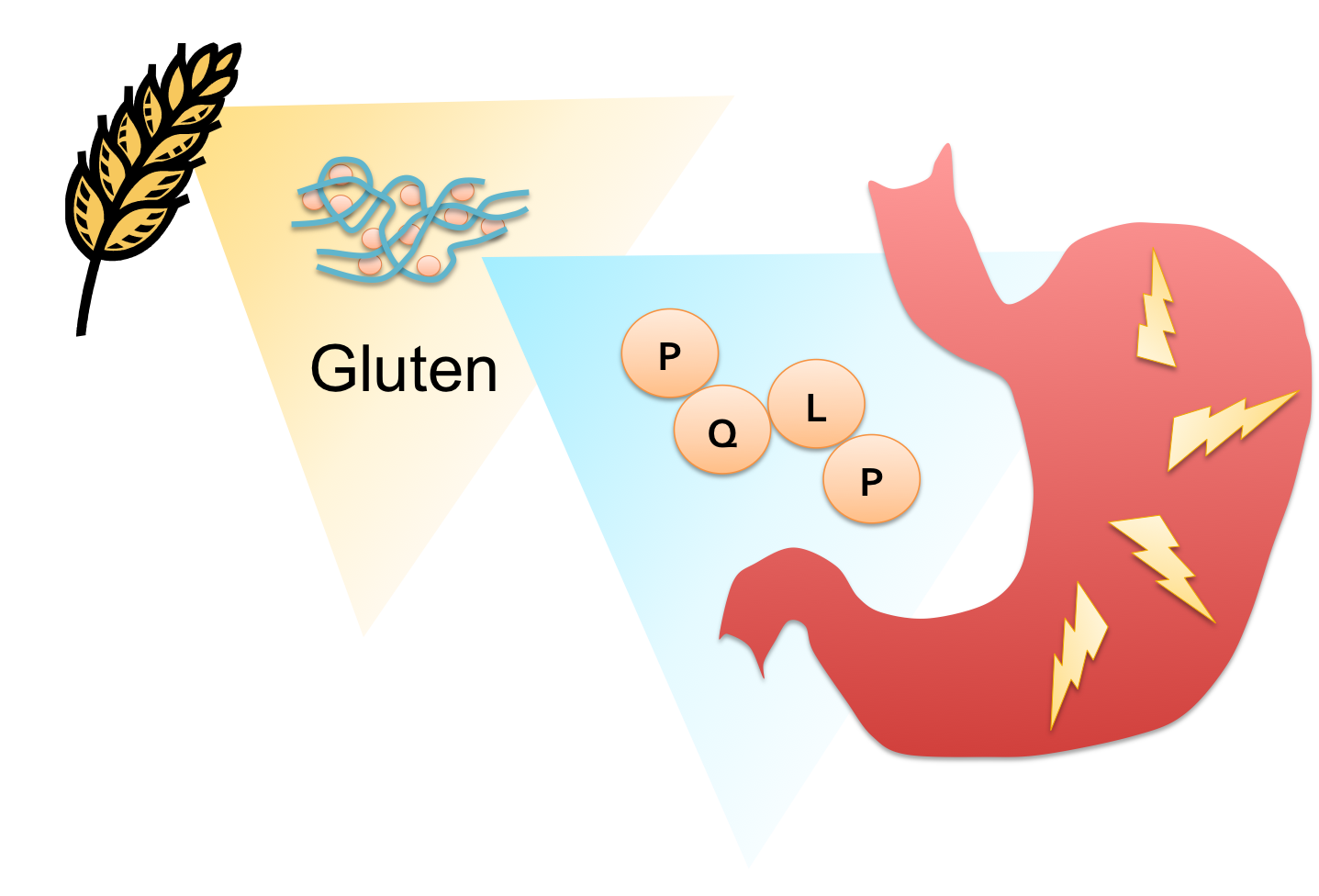
| + | [[File:Washington CD Diagram.png|right|250px|thumb|PQ rich regions of gluten cause painful inflammation in the digestive tract of people with Celiac Disease]] | ||
| + | |||
Celiac sprue is a highly prevalent disease in which dietary proteins found in wheat, barley, and rye products known as ‘glutens’ evoke an immune response in the small intestine of genetically predisposed individuals. The resulting inflammation can lead to the degradation of the villi of the small intestine, impeding the absorption of nutrients. Symptoms can appear in early childhood or later in life, and range widely in severity, from diarrhea, fatigue and weight loss to abdominal distension, anemia, and neurological symptoms. There are currently no effective therapies for this lifelong disease except the total elimination of glutens from the diet. Although celiac sprue remains largely underdiagnosed, its prevalence in the US and Europe is estimated at 0.5-1.0% of the population. With this in mind, we set out to design an enzyme therapeutic for gluten intolerance that could be taken in pill form. | Celiac sprue is a highly prevalent disease in which dietary proteins found in wheat, barley, and rye products known as ‘glutens’ evoke an immune response in the small intestine of genetically predisposed individuals. The resulting inflammation can lead to the degradation of the villi of the small intestine, impeding the absorption of nutrients. Symptoms can appear in early childhood or later in life, and range widely in severity, from diarrhea, fatigue and weight loss to abdominal distension, anemia, and neurological symptoms. There are currently no effective therapies for this lifelong disease except the total elimination of glutens from the diet. Although celiac sprue remains largely underdiagnosed, its prevalence in the US and Europe is estimated at 0.5-1.0% of the population. With this in mind, we set out to design an enzyme therapeutic for gluten intolerance that could be taken in pill form. | ||
Revision as of 19:02, 13 September 2011
Protein engineering of the peptidase Kumamolisin-As for use in treating gluten intolerance
Introduction
Celiac sprue is a highly prevalent disease in which dietary proteins found in wheat, barley, and rye products known as ‘glutens’ evoke an immune response in the small intestine of genetically predisposed individuals. The resulting inflammation can lead to the degradation of the villi of the small intestine, impeding the absorption of nutrients. Symptoms can appear in early childhood or later in life, and range widely in severity, from diarrhea, fatigue and weight loss to abdominal distension, anemia, and neurological symptoms. There are currently no effective therapies for this lifelong disease except the total elimination of glutens from the diet. Although celiac sprue remains largely underdiagnosed, its prevalence in the US and Europe is estimated at 0.5-1.0% of the population. With this in mind, we set out to design an enzyme therapeutic for gluten intolerance that could be taken in pill form.
Proline (P)- and glutamine (Q)-rich components of gluten known as ‘gliadins’ appear to be responsible for the bulk of the immune response int most patients. Their high PQ content protects gliadin oligopeptides from degradation by gastrointenstinal endoproteases, but also presents a target for drug design. Any peptidase capable of cleaving at or near the P-Q bond while remaining active at the temperature and harsh pH of the stomach would have pharmacological potential as a therapy for celiac sprue. One candidate enzyme, currently in clinical trials, utilizes a prolyl endopeptidase (PEP) from Sphingomonas capsulata (SC) to hydrolyze gliadins. This enzyme was a logical drug candidate as it has a native specificity for the proline rich gliadin peptides. Unfortunately, the enzyme’s optimal activity is at pH 7, and engineering attempts to enhance its activity at relevant gastric pH levels has not yet met with significant success. It is therefore pertinent to identify new candidate enzymes that have both activity on the gliadin PQ structural motif, as well as optimal activity at gastric pH levels during digestion- around pH 4.
Here, we describe an alternative approach to identifying and engineering an enzyme therapeutic for celiac sprue disease. Rather than focusing primarily on substrate specificity when choosing our candidate enzyme, we identified an enzyme already capable of catalyzing peptide hydrolysis at gastric pH levels, regardless of peptide substrate specificity. Upon identification of such an enzyme we used computational tools to reengineer its substrate specificity for enhanced activity on immunogenic gliadin peptides with the common PQ structural motif.
Enzyme Identification
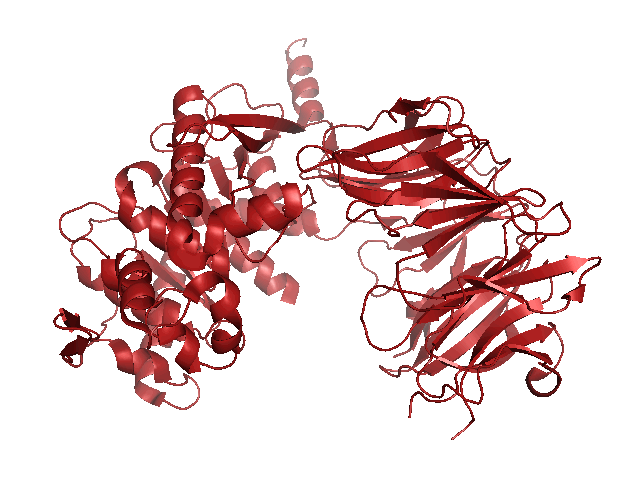
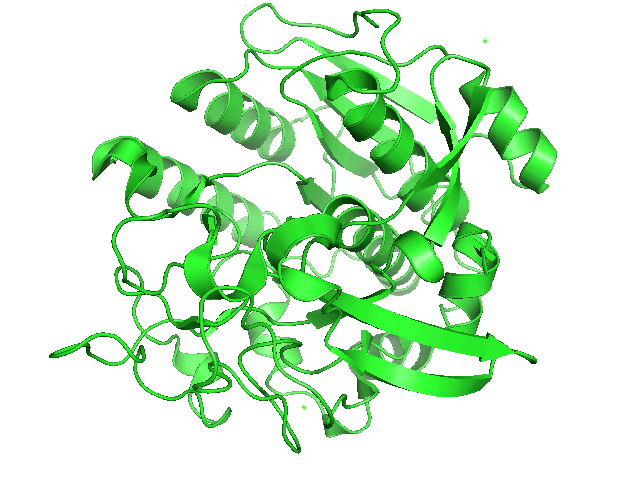
When searching for a protease with optimal activity at gastric pH levels that could be produced in a recombinant host, we identified an enzyme known as Kumamolisin-As. Kumamolisin-As was isolated from the thermoacidophilic bacterium Alicyclobacillus sendaiensis strain NTAP-1, has been shown to be produced in a recombinant host, and exhibits significant enzymatic activity from pH 2.5 and above. Its maximal activity is at pH ~4.0. It is this robust activity under acidic conditions that makes Kumamolisin-As so promising for the development of a pill for gluten intolerance, since it would remain active at the harsh pH of the stomach.
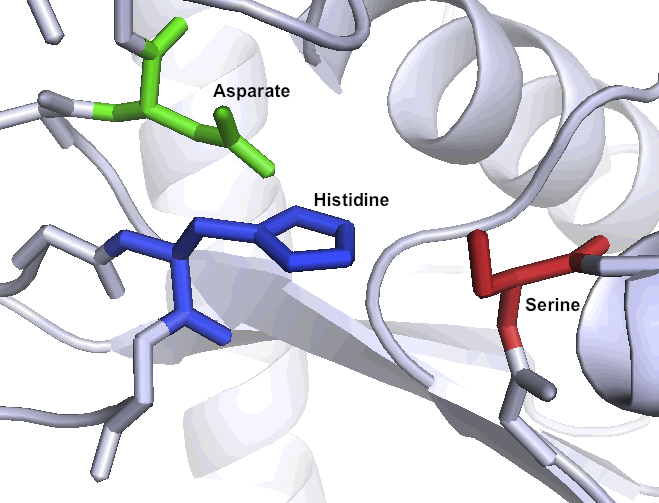
One of the primary reasons that Kumamolisin-As is so much more active than SC-PEP at low pH is due its catalytic triad, the three amino acid residues most heavily involved in conducting the chemistry of cleaving peptides. SC-PEP is part of a large class of enzymes known as serine proteases, which make use of the catalytic triad Serine-Histidine-Aspartate. Acidic conditions can impede the histidine's ability to play its role in this triad, making most serine proteases vulnerable to low pH. Kumamolisin-As, on the other hand, is a member of the sedolisin family of serine-carboxyl peptidases, which utilize the key catalytic triad Ser278-Glu78-Asp82 to hydrolyze their substrates. It is the acidic Glutamate, as opposed to Histidine, in the triad that makes Kumamolisin-As able to catalyze peptide cleavage at low pH. While the native substrate for this enzyme is unknown, it has been previously shown to degrade collagen under acidic conditions. In addition, this enzyme has been shown to be thermostable, with an ideal temperature at 60ºC, but still showing significant activity at 37ºC.
Specificity studies have also been done, and the enzyme is most stringent at the P1 and P2 subsites, the ideal amino acids being arginine and proline, respectively. The combination of Kumamolisin-As having the desired enzymatic activity under gastric pH levels, and the P2 subsite already having the specificity for the proline from the desired PQ motif makes it a highly attractive candidate for further engineering. In addition, several crystal structures of this enzyme have been solved, allowing for the use of computational design techniques.
References
1.Shan, Lu, et al. "Structural Basis for Gluten Intolerance in Celiac Sprue." Science 297.5590 (2002): 2275-2279.
2.Mustalahti, Kirsi, et al. "The prevalence of celiac disease in Europe: Results of a centralized, international mass screening project." Annals of Medicine 42.8 (2010): 587-595.
3.Ehren, Jennifer, et al. "Protein engineering of improved prolyl endopeptidases for celiac sprue therapy." Protein Engineering, Design & Selection 21.12 (2008): 699-707.
4.Wlodawer, Alexander, et al. "Crystallographic and Biochemical Investigations of Kumamolisin-As, a Serine-Carboxyl Peptidase with Collagenase Activity." The Journal of Biological Chemistry 279.20 (2004): 21500-21510.
 "
"