Team:Wageningen UR/Project/Devices
From 2011.igem.org
Matthijnwur (Talk | contribs) (→Custom fluidic device designed by Team Wageningen UR to measure oscillations) |
Matthijnwur (Talk | contribs) (→Custom fluidic device designed by Team Wageningen UR to measure oscillations) |
||
| Line 53: | Line 53: | ||
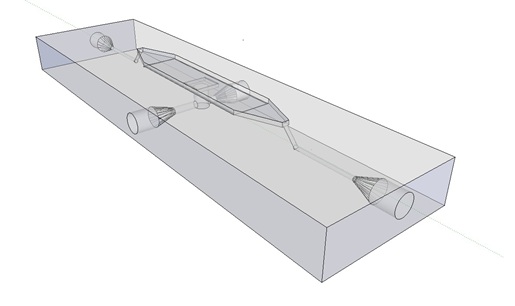
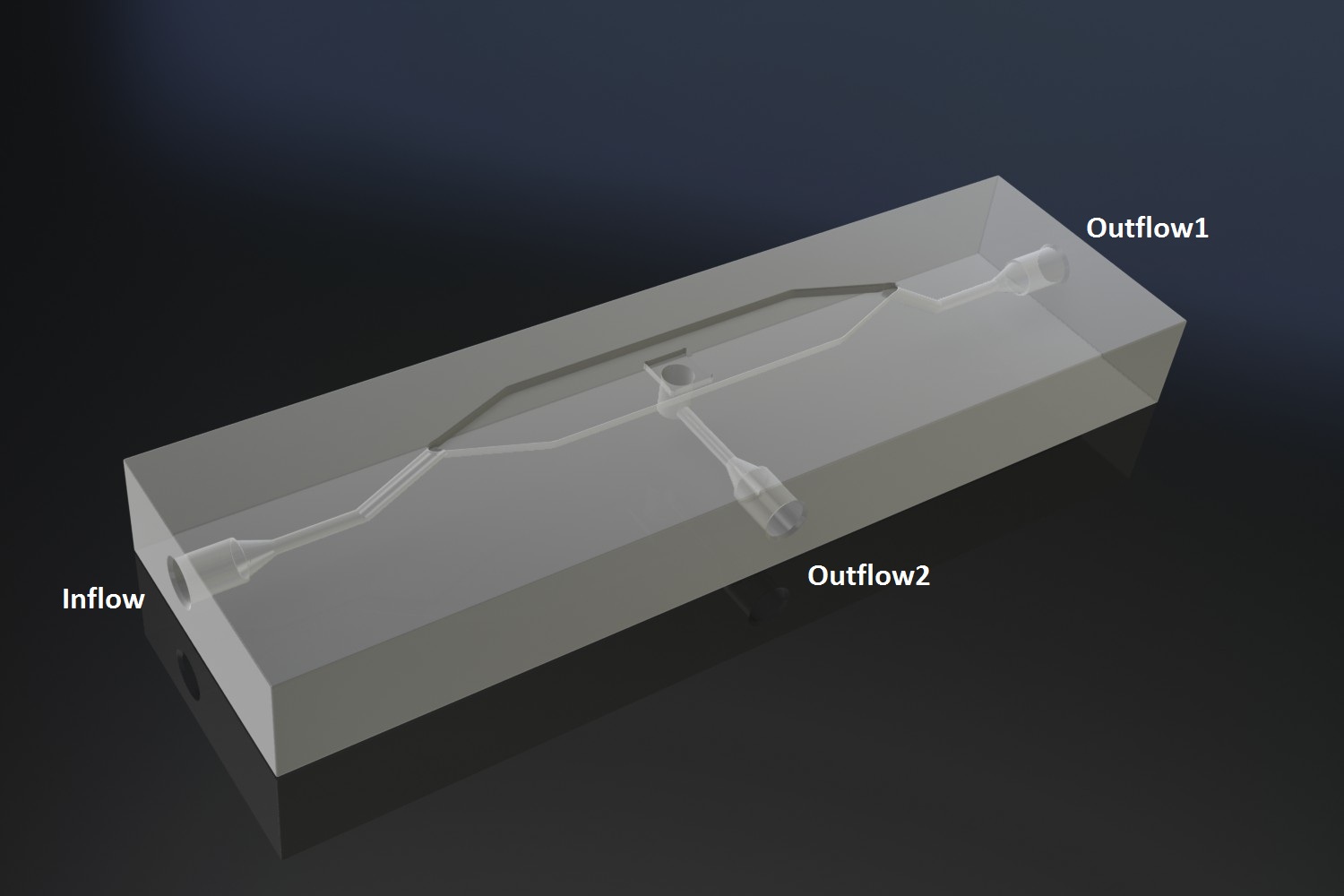
Potentially inducing and observing oscillatory behaviour in a population of e.coli cells with the means of using a micro-sieve, a cake of cells should be formed on the membrane. In dairy industry they do this by applying the filtrate through an inflow port leading to a chamber, which houses the micro-sieve. Because an overpressure is produced in the chamber liquid will be forced through the sieve, and the suspended particles in our case e.coli cells will aggregate on the sieve. However to prevent that the pressure in the chamber reaches a too high pressure that cells get lysed by being pushed through the filter also an outflow port is included. In the figure below the functional components of the flow device are depicted. | Potentially inducing and observing oscillatory behaviour in a population of e.coli cells with the means of using a micro-sieve, a cake of cells should be formed on the membrane. In dairy industry they do this by applying the filtrate through an inflow port leading to a chamber, which houses the micro-sieve. Because an overpressure is produced in the chamber liquid will be forced through the sieve, and the suspended particles in our case e.coli cells will aggregate on the sieve. However to prevent that the pressure in the chamber reaches a too high pressure that cells get lysed by being pushed through the filter also an outflow port is included. In the figure below the functional components of the flow device are depicted. | ||
| + | |||
| + | [[File:innerdevice.jpg]] | ||
| + | |||
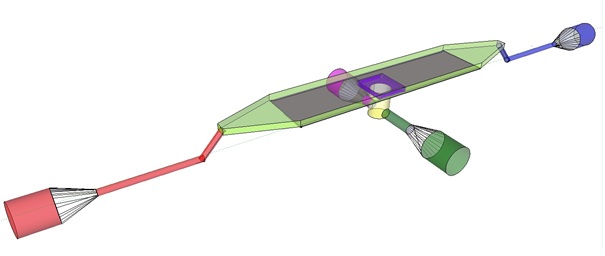
| + | '''Fig.4:''' ''Functional components flow device: In red the inflow port and channel to the top chamber of the flow device. In light green the flow chamber containing either in black the micro-dish or in purple the micro-sieve. In blue the outflow port of the top chamber. In yellow the bottom chamber with it respective in and out flow channels and ports.'' | ||
| + | |||
| + | In our flow device outfitted with the micro sieve the inflow is depicted in red, the chamber in light green, outflow in blue and the through flow ports in green or pink. | ||
| + | |||
| + | For potentially inducing and observing oscillatory behaviour in a population of e.coli cells with the means of using a micro-dish. Nutrients should be readily available for the cells located in the wells. To achieve this the design implements a second set of inflow (in green) and outflow(in pink) ports, these ports connect to a chamber when filled with medium comes in contact with the aluminium oxide micro-dish(in black). Through the addition of these ports an continuous supply of fresh medium is supplied. | ||
| + | |||
| + | '''General design considerations.''' | ||
| + | |||
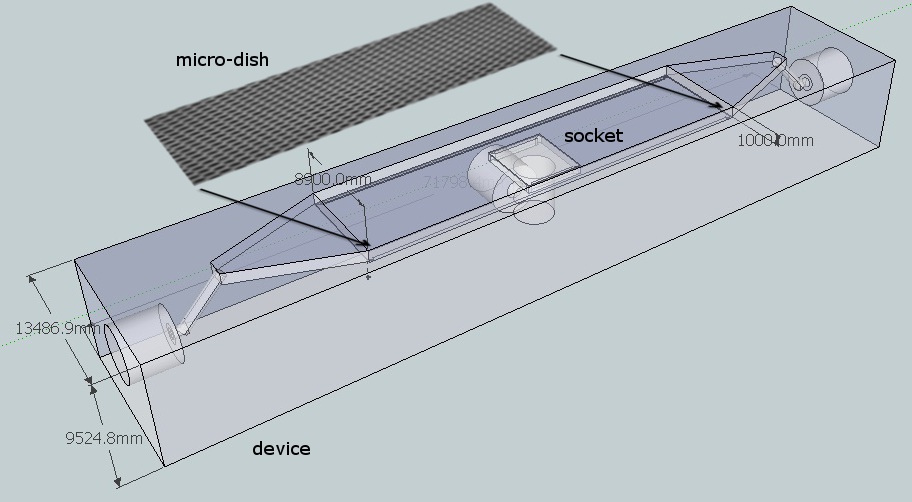
| + | Because we want to use the flow device in combination with fluorescence microscopy the distance between the objective and the sample is crucial. Due to this fact we choose for a top chamber depth of 1 mm because in combination with the deckled of 1 mm, this distance is still short enough for the focusing depth of the 20 x objective. Furthermore this also reduces the volume of the chamber, reducing overhead liquid which could contain precious reactants ,such as, acyl-homo-lactone. Another general design consideration was that we wanted to have an even flow over the microsieve. That,s why the in and out flow side of the top chamber are triangular because this will reduce flow differences over the sieve. | ||
| + | |||
| + | |||
Revision as of 19:54, 19 September 2011
 "
"