Team:USTC-China/Drylab/riboswitch
From 2011.igem.org

Contents |
Abstract
Riboswitches are RNA-based genetic control elements that regulate gene expression in a ligand-dependent fashion without the need for proteins. Herein, we collect theophylline aptamers and their molecular analogues aptamers including adenine, xanthine, 3-methylxanthine, hypoxanthine and caffeine. All these sequences are available in supplemental data. We want to find out correlations among those aptamer sequences and build versatile expression platforms for required aptamer. In order to achieve these goals, we classify the aptamer sequences by analyzing their secondary structures and identify the consensus sequences by sequence alignment (BLAST engine).Finally, we reveal the riboswitches function and structure relationship and suggest design principles for creating new synthetic riboswitches and their platforms.
Analysis
Theophylline
Classify
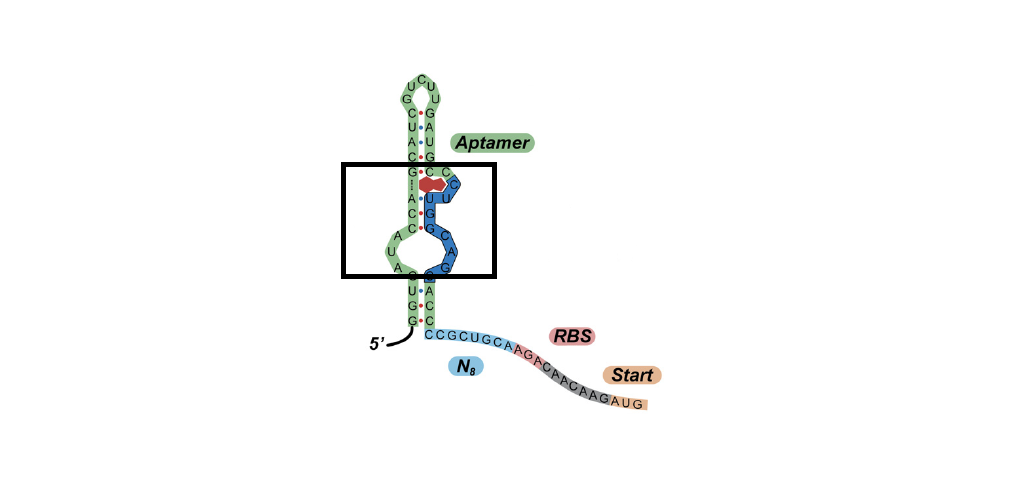
First, we predicted the secondary structure of the theophylline aptamers by mfold. Then we found that the aptamers can be classified into 3 categories by their secondary structure shapes. The first category aptamers have two loops, the second category aptamers have three loops and the last category aptamers have complex secondary structures which cannot be classified by the number of loops (Figure 1).
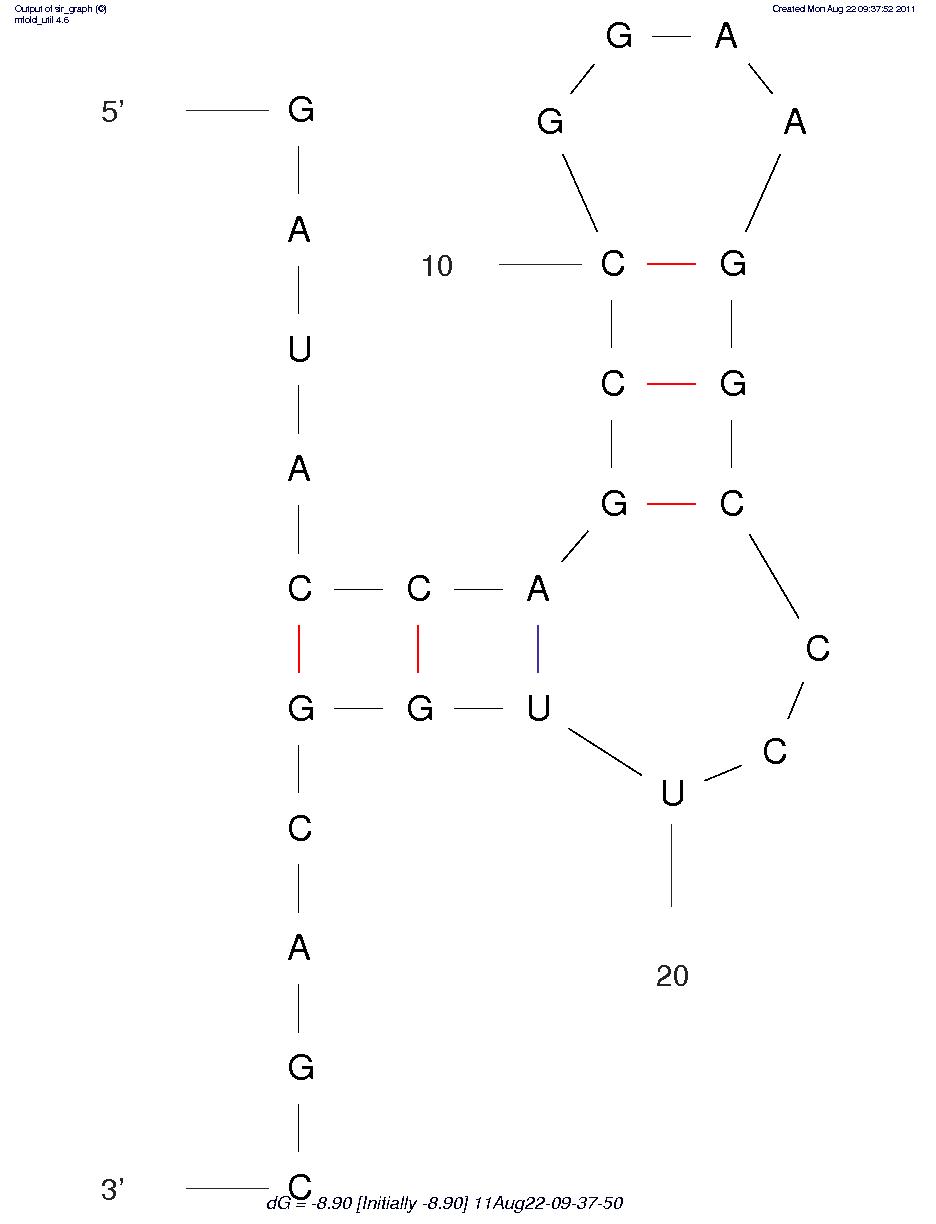
Figure 1A. One of the category 1 aptamer secondary structure
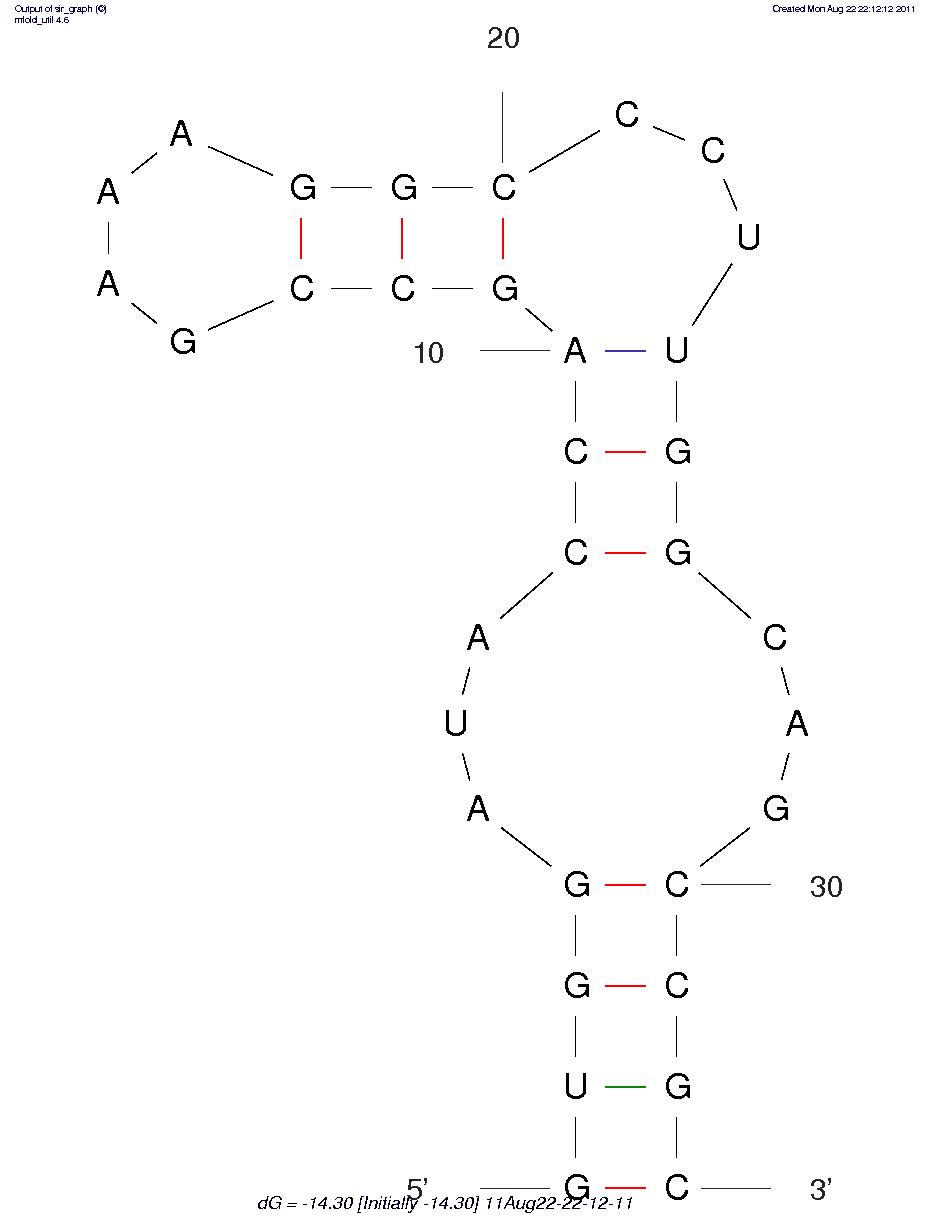
Figure 1B.One of the category 2 aptamer secondary structure
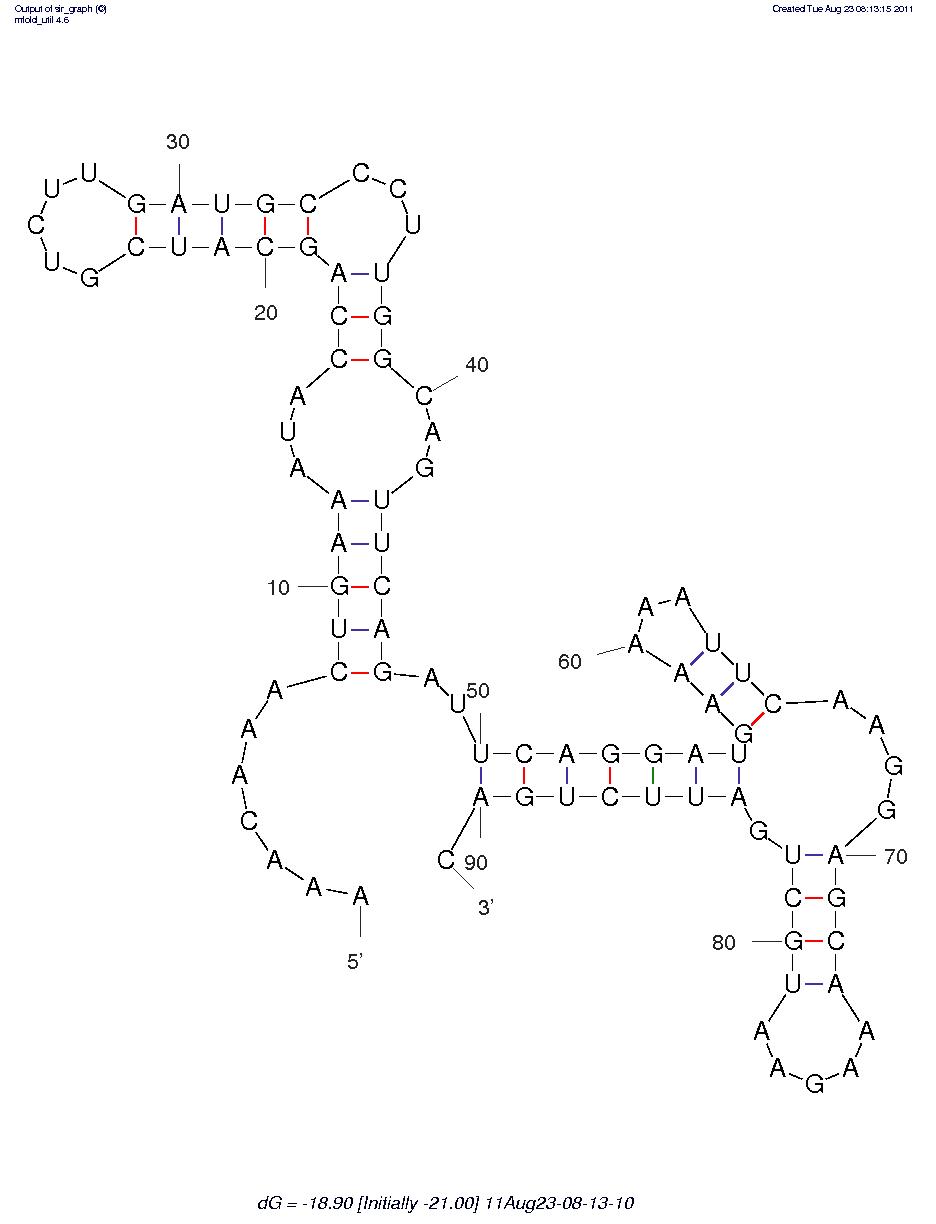
Figure 1C.One of the category 3 aptamer secondary structure
Sequence alignment
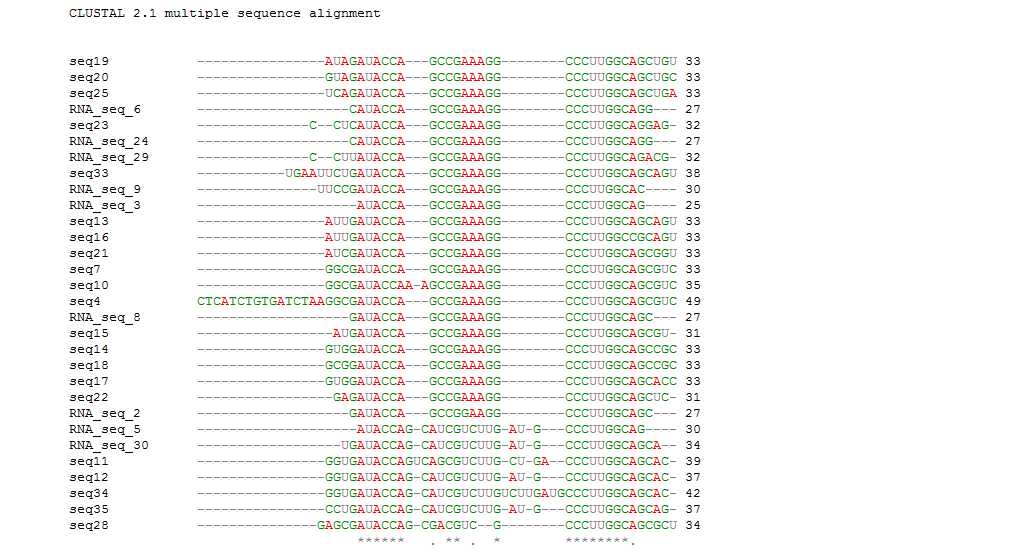
We previously got the secondary structures of the theophylline aptamers and classified those aptamer sequences into 3 categories. To find out the consensus sequences, we did the sequence alignment in each categories by Clastalw2 and the alignment indicated that the conserved sequences have varied length in different groups (Table 1)(Figure 2B). The alignment results are available in the supplemental data.
TABLE 1. Consensus sequences finds in sequences alignment
| Consensus sequence1 | Consensus sequence1 | |
| Consensus sequence1 | AUACCAGC | GCCCUUGGCA |
| Category2 | AUACCAG | GCCCUUGGCA |
| Category3 | CG | CUUGGCA |
| Whole sequences | C | GG |
| Whole sequences(except seq26, seq27,seq31,seq32) | AUACCA | CCCUUGGC |
Then we compared the whole aptamer sequences and their conserved sequences. From this alignment, we identified that the conserved sequences have the same location in the whole aptamers and the aptamers do have the same consensus sequences except several complex long aptamers. Notably, the previous work by Gallivan has reported the same motif sequences (Figure 2).
Figure 2A.The two motifs have been reported by Gallivan.
A High-Throughput Screen for Synthetic Riboswitches Reveals Mechanistic Insights into Their Function DOI 10.1016/j.chembiol.2006.12.008
Figure 2B. The whole sequences except 26,27,31,32 alignment result
Phylogenetic analysis
The guide tree is a branching diagram(tree), assumed to be an estimated of a phylogeny, branch lengths are proportional to the amount of inferred evolutionary change (Figure 3).
From the guide tree diagram 1 and 2, we found that the sequences are similar to each other except sequence 5 and sequence 30. Actually, the sequences alignment also indicates the sequences have relatively long consensus sequences. In guide tree diagram 3, there are less sequences than diagram 1 and 2. And the consensus sequences are quite short since the comparatively long sequences in diagram 3 are different, especially sequence 27.
From the whole sequence guide tree diagram, we found that sequences in category 1 are mixed with sequences in category 2. Thus, the classification by the sequences secondary structure may not restrict in the phylogenetic analysis perspective. The sequence 27 which is different from the other sequences in diagram 3 shows evolutionary change in the whole sequences diagram.
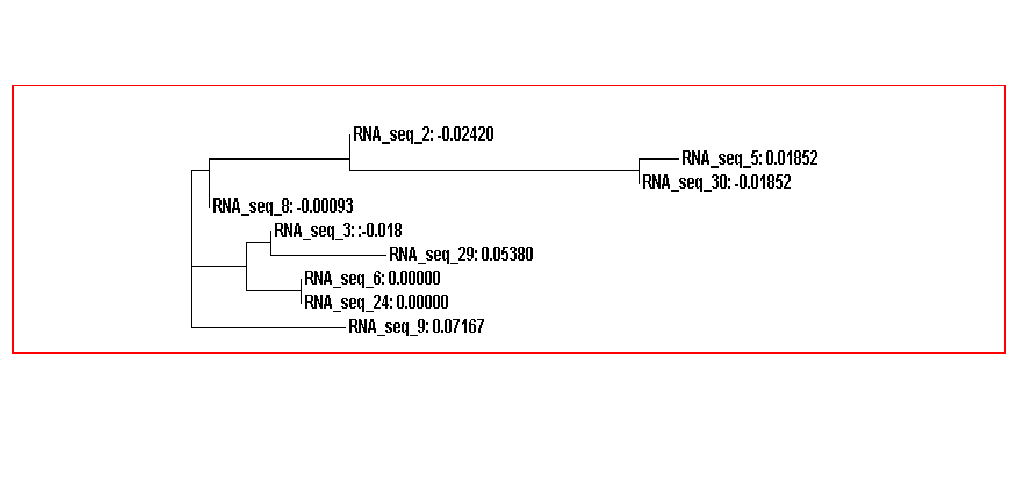 Figure 3A. Guide tree 1
Figure 3A. Guide tree 1
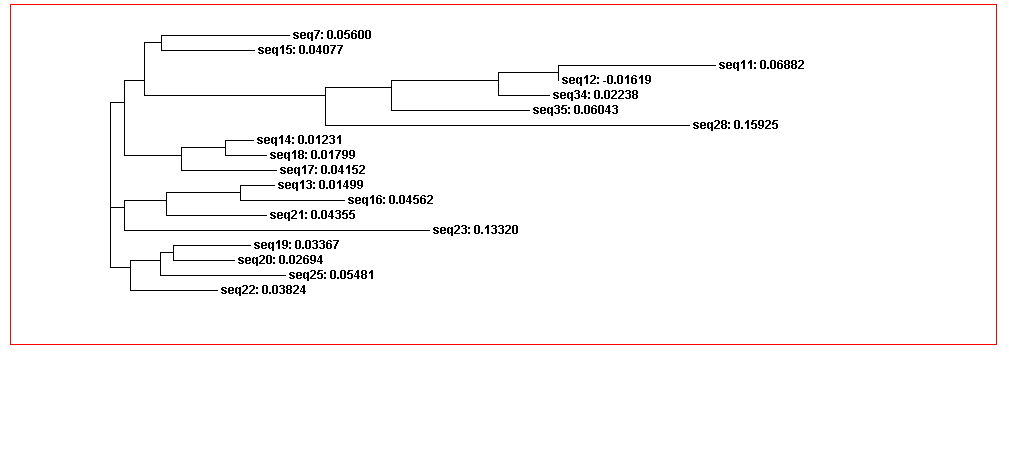 Figure 3B. Guide tree 2
Figure 3B. Guide tree 2
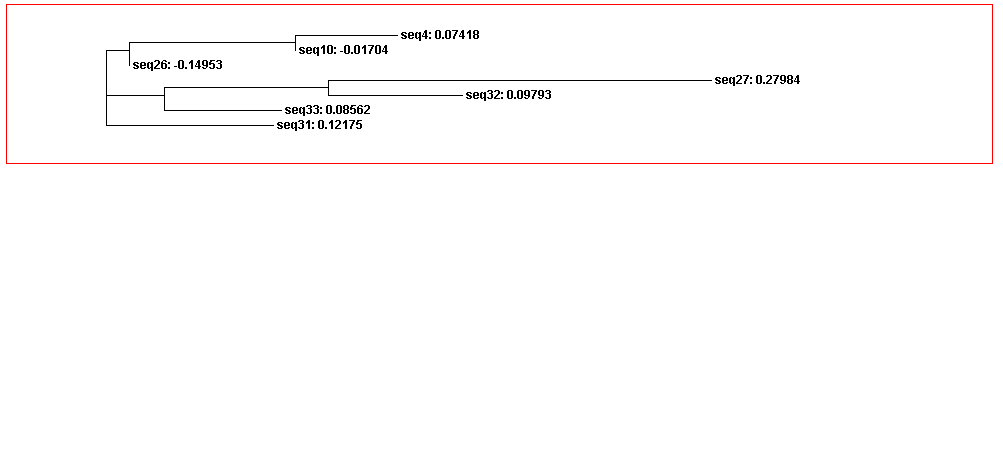 Figure 3C. Guide tree 3
Figure 3C. Guide tree 3
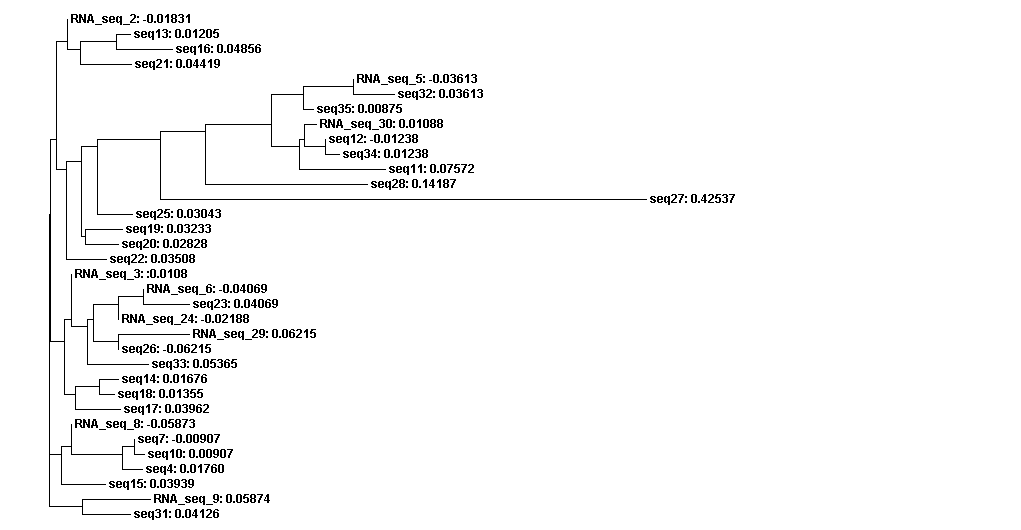 Figure 3D. Whole sequences guide tree
Figure 3D. Whole sequences guide tree
Secondary structure and free energy
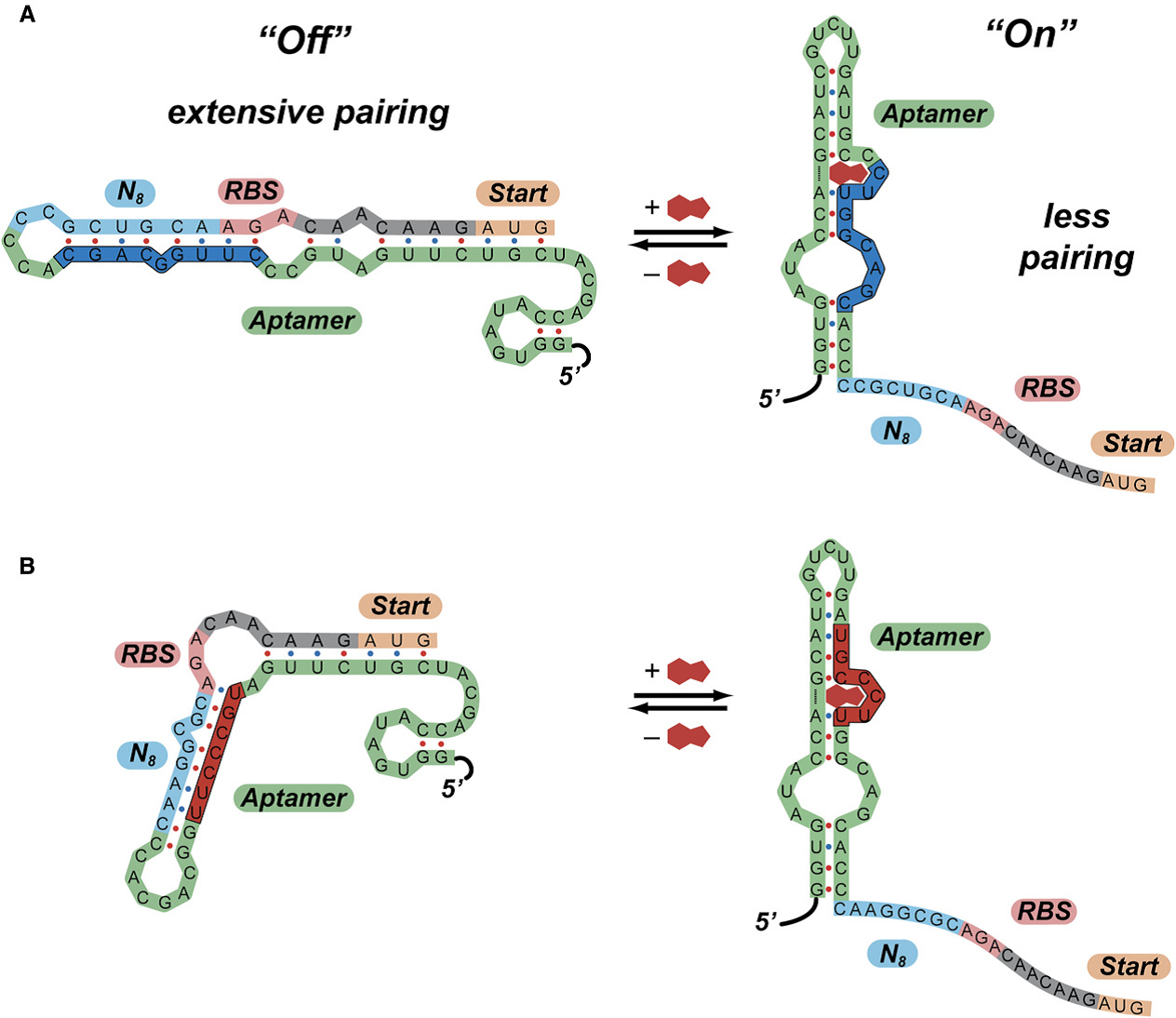
The previous work by Gallivan has shown that the aptamers usually have two conformations with different free energy and the presence of the theophylline would change the equilibrium state which the aptamers secondary structures would shift from ‘’off’’ state to ‘’on’’ state (Figure 4). The possible switching mechanism in which extensive paring in the region near the ribosome binding site prevents translation of downstream genes have been demonstrated by de Smit and van Duin.
Therefore we reconsider our previous work and the result suggests that only relative long aptamer sequence would fold to different conformation. Besides, the longer the sequences are the lower free energy the aptamer would have. And the most aptamer sequences we have collected are only the target sense section which can bind the target molecule but cannot fold into different conformations. That is, we should design the action section to accomplish the aptamer’s function.
Combing to the sequence alignment, we find that the consensus sequences locates in the region which would interact with the target molecule. As we expected, the results suggests the ligand-binding conform to the structure –function relationship.
Figure 4. Predicted Mechanisms of Action of Synthetic Riboswitches
A High-Throughput Screen for Synthetic Riboswitches Reveals Mechanistic Insights into Their Function DOI 10.1016/j.chembiol.2006.12.008
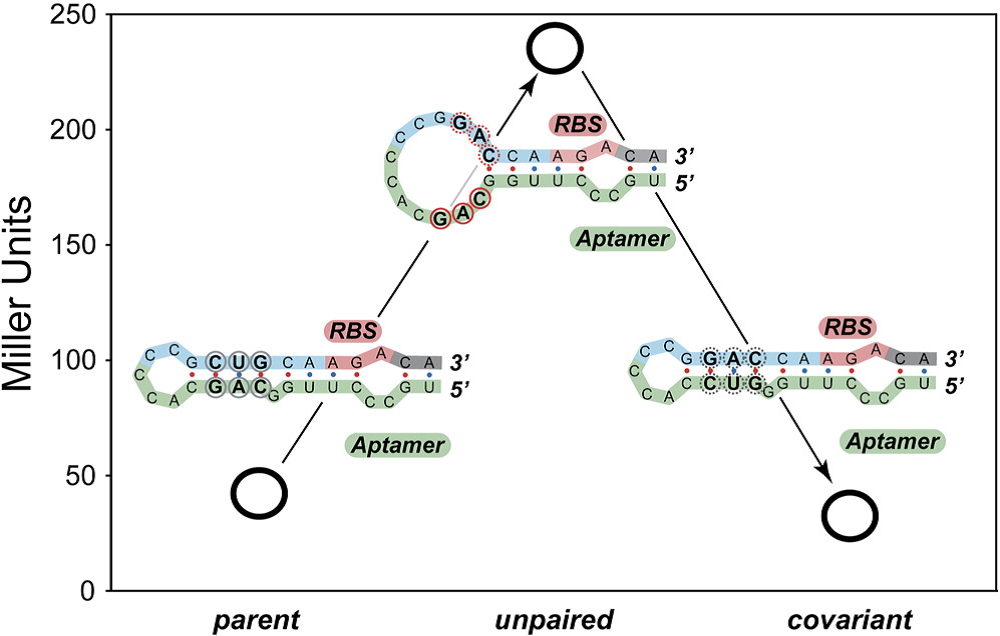
The covariance experiment by Gallivan indicates that base-pairing between the aptamer and a region near the ribosome binding would decrease the upstream gene expression which would be used to improve the aptamers’ performance (Figure 5). Notably, we use the mfold to predict the secondary structure of the aptamer which has been reported by Gallivan but cannot get the expected two varied conformation.
Figure 5. Results from Covariance Experiments
A High-Throughput Screen for Synthetic Riboswitches Reveals Mechanistic Insights into Their Function DOI 10.1016/j.chembiol.2006.12.008
Adenine
Classify
As the same to the theophylline, we use mfold to predict the secondary structure of the adenine. From the results, we classified the aptamer sequences into 2 categories by their secondary structure. The sequences in the first category have the same secondary structures which have been usually reported and studied as the adenine apatamers. The sequences in the second category have varied conformation and owing to their relatively long sequence, they can fold to complex conformations.
Sequence alignment
To locate the consensus sequences and probe correlation between adenine apatmer sequences and theophylline aptamer sequences, we did sequence alignment in each categories and whole sequences (Table 2) (Figure7) . From the alignment result, we found that the sequences in category 2 are quite different and have relative short consensus sequences. We thought the data which have been collected are not well processed.
We compared the adenine aptamer sequences alignment results with theophylline, we found that there are more consensus sequences locating in different region and the entire adenine aptamer sequence show complex structure. Thus, the comparison indicates that adenine aptamer sensor domain and theophylline have less or nothing in common.
In addition, the adenine alignment has shown the same consensus sequences as Daniel A. Lafontaine reported(Figure 8) Who has pointed out the importance position in adenine aptamer sequences.
TABLE 2. Consensus sequences finds in sequences alignment Consensus sequence1 Consensus sequence2 Consensus sequence3 Category1 UAUAA GU UGAUUA Category2 U U UUA Whole sequences A \ UU Whole sequences except 36,37,38,41,42,53 A UU AUU
The consensus sequences 1,2,3 locate around 15,39,65 reported by Daniel A. Lafontaine
Core requirements of the adenine riboswitch aptamer for ligand binding RNA 2007 13: 339-350 originally published online January 2, 2007
Phylogenetic analysis
Using Clustalw2, we could describe adenine aptamer sequences evolution change. Thus, sequences in category 1 more similar than category 2 since the sequences in category 1 have the same secondary structures. However, the sequences in category 1 and 2 mixes in the whole sequences guide trees. Since the adenine aptamers have completely different secondary structures from theophylline, we didn’t compare adenine aptamer with theophylline.
Secondary structure and free energy
Two representatives of the adenine aptamers have been reported (Figure10). One is ydhL A-riboswitch and the other is add A-riboswitch. Both of them have the same secondary structure which have been demonstrated by our previous prediction (Figure 6A).
A remarkable feature of the ligand bound scaffolds is the formation of a junctional core involving five stacked triples (Ronald R. Breaker,3 and Dinshaw J. Patel1et al.2004). The crystal structures show that the bound ligand is directly involved in a Watson-Crick base pair at position 65 making this residue particularly important. Our sequence alignment also indicates that position 65 is consensus sequence.
<[>Notably, the structure-function relationship in adenine aptamer would be more precisely described by their crystal structure nut not the secondary structure. In fact, we cannot predict the two conformation of the adenine aptamer by mfold.</p>
Reference
1.Shana Topp and Justin P. Gallivan. 2006. Guiding Bacteria with Small Molecules and RNA. Jacs.
2.Sean A. Lynch, Shawn K. Desai, Hari Krishna Sajja, and Justin P. Gallivan. 2007. A High-Throughput Screen for Synthetic Riboswitches Reveals Mechanistic Insights into Their Function. Cell.
3. de Smit, M.H., and van Duin, J. 1990. Secondary structure of the ribosome binding site determines translational efficiency: a quantitative analysis. Proc. Natl. Acad. Sci. USA 87, 7668–7672.
4. de Smit, M.H., and van Duin, J. 1994. Control of translation by mRNA secondary structure in Escherichia coli. A quantitative analysis of literature data. J. Mol. Biol. 244, 144–150.
5.de Smit, M.H., and van Duin, J. (1994). Translational initiation on structured messengers. Another role for the Shine-Dalgarno interaction. J. Mol. Biol. 235, 173–184.
6. Jean-FranÇois Lemay and Daniel A. Lafontaine. 2007. Core requirements of the adenine riboswitch aptamer for ligand binding. RNA
7. Alexander Serganov, Yu-Ren Yuan,Olga Pikovskaya,1 Anna Polonskaia, Lucy Malinina,1 Anh Tuân Phan, Claudia Hobartner, Ronald Micura, Ronald R. Breaker, and Dinshaw J. Patel. 2004.Chem. Biol.
 "
"