Team:UNAM-Genomics Mexico/Notebook/nifH
From 2011.igem.org
| Line 31: | Line 31: | ||
We transformed competent cells with parts J23109, J23112, J23113, J23114, J23115 and I20260 because the first transformations performed with these parts were not successful. Unfortunately not all of these transformations were successful so we repeated them for a third time, this time increasing the amount of DNA with which we performed the transformation and at last we got good results. By the end of this week we has transformed competent bacteria with every part from the Anderson promoter collection as well as parts I20260 and E0240. We divided the Anderson promoter collection in two: the first part includes parts J23100 to J23111 and the second part includes parts J23112 to J23119. | We transformed competent cells with parts J23109, J23112, J23113, J23114, J23115 and I20260 because the first transformations performed with these parts were not successful. Unfortunately not all of these transformations were successful so we repeated them for a third time, this time increasing the amount of DNA with which we performed the transformation and at last we got good results. By the end of this week we has transformed competent bacteria with every part from the Anderson promoter collection as well as parts I20260 and E0240. We divided the Anderson promoter collection in two: the first part includes parts J23100 to J23111 and the second part includes parts J23112 to J23119. | ||
| + | Week: July 11- July 15 | ||
| + | Once we had E. coli transformed with all of the parts that we wanted, we started to extract plasmids from both the first and the second part of the promoter collection as well as from parts E0240 and I20260. We did this because our focus was to obtain as much clean plasmid from each part so we could later digest them with EcoRI and PstI and isolate the parts from an agarose gel. | ||
| + | |||
| + | [[File:Unamgenomicsfig1nifh.jpg|center|frame|Figure 1. Example of the low-quality plasmid extractions]] | ||
}} | }} | ||
Revision as of 06:39, 28 September 2011
Contents |
Lab Logbook - Rhizobium etli kit
The objective of this section is to explain the experimental strategies and activities that we undertook in order to achieve the following objectives:
- Characterization of the promoter region of the nifHa gene of Rhizobium etli. The characterization involves describing the strength of this promoter region by comparing the expression GFP and RFP reporters under this promoter to the expression from a reference promoter from the iGEM Registry of parts, J23101.
- Characterization of the Anderson promoter collection for use in Rhizobia species. The aim is to describe the strength of the promoters that constitute this collection in our chasis, Rhizobium etli.
The activities are organized by weeks (monday to sunday); we mention the general remarks for the activities that we performed.
Week: June 13 - June 19
This week we amplified the promoter region from the nifHa gene of Rhizobium etli. We amplified this region by using primers that contained the prefix and suffix sequences in upper and lower primers respectively so that when we amplified the promoter region, we would end up with the prefix and suffix flanking the promoter region. We cleaned the PCR product and later assessed the size of the amplified product by gel electrophoresis. We saw that the size of amplified region did correspond to the size of the promoter region which is 252 bp (foto impresa).
Week: June 27 - July 1st
This week we started by transforming four parts from the iGEM Spring 2011 Distribution, parts: J23101, J23102, E0240, and I20260. Parts J23101 and J23102 belong to the Anderson promoter collection, E0240 is a GFP generator, and I20260 is a measurement device for the promoter J23101. We achieved successful transformation for J23101, J23102, and E0240. We did these transformations to prove if the protocol we were going to use was fine. Since we had successful results, we continued with the transformation of the rest of the promoters in the Anderson promoter collection.
Week: July 4 - July 8
We transformed competent cells with parts J23109, J23112, J23113, J23114, J23115 and I20260 because the first transformations performed with these parts were not successful. Unfortunately not all of these transformations were successful so we repeated them for a third time, this time increasing the amount of DNA with which we performed the transformation and at last we got good results. By the end of this week we has transformed competent bacteria with every part from the Anderson promoter collection as well as parts I20260 and E0240. We divided the Anderson promoter collection in two: the first part includes parts J23100 to J23111 and the second part includes parts J23112 to J23119.
Week: July 11- July 15
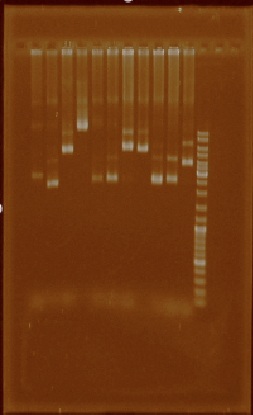
Once we had E. coli transformed with all of the parts that we wanted, we started to extract plasmids from both the first and the second part of the promoter collection as well as from parts E0240 and I20260. We did this because our focus was to obtain as much clean plasmid from each part so we could later digest them with EcoRI and PstI and isolate the parts from an agarose gel.
 "
"