Team:UCL London/Medicine/DNAVaccines/Methods
From 2011.igem.org
(Created page with "{{:Team:UCL_London/Template/Header}}<html> <div id="submenu" class="sm-medical-dnavaccines"> <div id="medical-dnavaccines2"><a href="https://2011.igem.org/Team:UCL_London/Medicine...") |
|||
| Line 6: | Line 6: | ||
<h1>Viral / Other non egg-based methods comparison</h1> | <h1>Viral / Other non egg-based methods comparison</h1> | ||
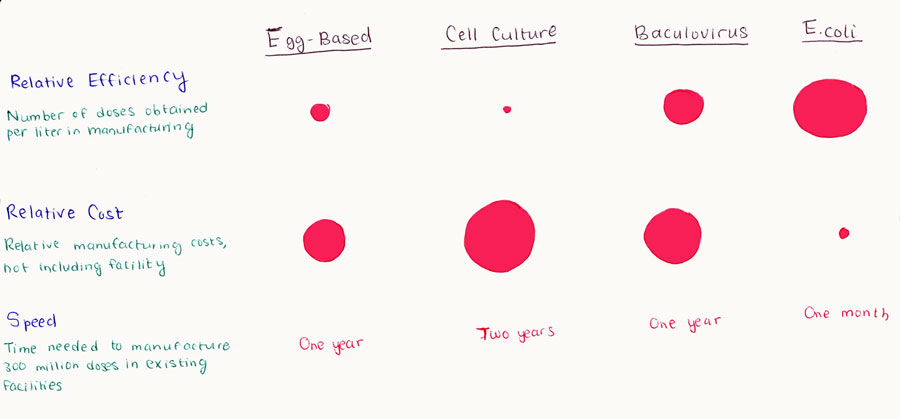
| - | There are a great deal of advantages to using the baculovirus expression system for vaccine development. This includes its “high expression levels, limitless size of expressed protein, efficient cleavage of signal peptides, processing of the protein and post-translational modifications, simultaneous expression of multiple genes and short time for protein expression. BEVS can be used with a wide variety of transfer vectors as well. A drawback to this system is the high-titer viral stocks needed to infect cell culture stocks. Production of these stocks is a very laborious and time consuming process. Another setback is that the BEVS cell lines can´t be kept for very long otherwise there will be an increase in defective viral particles. | + | There are a great deal of advantages to using the baculovirus expression system for vaccine development. This includes its “high expression levels, limitless size of expressed protein, efficient cleavage of signal peptides, processing of the protein and post-translational modifications, simultaneous expression of multiple genes and short time for protein expression. BEVS can be used with a wide variety of transfer vectors as well. A drawback to this system is the high-titer viral stocks needed to infect cell culture stocks. Production of these stocks is a very laborious and time consuming process. Another setback is that the BEVS cell lines can´t be kept for very long otherwise there will be an increase in defective viral particles.”<sup>[[Team:UCL_London/Bibliography#medicine-15|[15]]]</sup> |
Cell-culture-based technology is robust and reliable and is a potential replacement for chicken-egg based vaccine production. Once the virus is propagated and harvested, the downstream processing parameters for purification, filling, and packaging of the vaccine are similar to current pharmaceutical methodologies and egg-based methodologies. However, this process is far less time consuming because of the use of cell lines. Once a cell line is infected with the seed virus in a fermenter, the process can begin. The most difficult issue is obtaining the seed virus. For mammalian cell expression it´s difficult to obtain a stable cell line, reasons behind this are high toxicity within the cell. | Cell-culture-based technology is robust and reliable and is a potential replacement for chicken-egg based vaccine production. Once the virus is propagated and harvested, the downstream processing parameters for purification, filling, and packaging of the vaccine are similar to current pharmaceutical methodologies and egg-based methodologies. However, this process is far less time consuming because of the use of cell lines. Once a cell line is infected with the seed virus in a fermenter, the process can begin. The most difficult issue is obtaining the seed virus. For mammalian cell expression it´s difficult to obtain a stable cell line, reasons behind this are high toxicity within the cell. | ||
| - | An advantage of using cell based manufacture is that the cell lines can be kept frozen indefinitely which makes it reasonably quick to produce vaccines in event of an epidemic.16 The cell-culture vaccine process is suitable for large-scale manufacture, and can be run routinely as well as cost effectively. The typical cell-culture production process can be run in batch sizes at scales that are sufficient to provide vaccine quantities either for interpandemic periods or even for pandemics. However, no vaccines have been licensed using this technology as yet. | + | An advantage of using cell based manufacture is that the cell lines can be kept frozen indefinitely which makes it reasonably quick to produce vaccines in event of an epidemic.<sup>[[Team:UCL_London/Bibliography#medicine-16|[16]]]</sup> The cell-culture vaccine process is suitable for large-scale manufacture, and can be run routinely as well as cost effectively. The typical cell-culture production process can be run in batch sizes at scales that are sufficient to provide vaccine quantities either for interpandemic periods or even for pandemics. However, no vaccines have been licensed using this technology as yet. |
[[File:Ucl-content-Medicine-eggchart.jpg|center]] | [[File:Ucl-content-Medicine-eggchart.jpg|center]] | ||
Latest revision as of 01:15, 22 September 2011
Viral / Other non egg-based methods comparison
There are a great deal of advantages to using the baculovirus expression system for vaccine development. This includes its “high expression levels, limitless size of expressed protein, efficient cleavage of signal peptides, processing of the protein and post-translational modifications, simultaneous expression of multiple genes and short time for protein expression. BEVS can be used with a wide variety of transfer vectors as well. A drawback to this system is the high-titer viral stocks needed to infect cell culture stocks. Production of these stocks is a very laborious and time consuming process. Another setback is that the BEVS cell lines can´t be kept for very long otherwise there will be an increase in defective viral particles.”[15]
Cell-culture-based technology is robust and reliable and is a potential replacement for chicken-egg based vaccine production. Once the virus is propagated and harvested, the downstream processing parameters for purification, filling, and packaging of the vaccine are similar to current pharmaceutical methodologies and egg-based methodologies. However, this process is far less time consuming because of the use of cell lines. Once a cell line is infected with the seed virus in a fermenter, the process can begin. The most difficult issue is obtaining the seed virus. For mammalian cell expression it´s difficult to obtain a stable cell line, reasons behind this are high toxicity within the cell.
An advantage of using cell based manufacture is that the cell lines can be kept frozen indefinitely which makes it reasonably quick to produce vaccines in event of an epidemic.[16] The cell-culture vaccine process is suitable for large-scale manufacture, and can be run routinely as well as cost effectively. The typical cell-culture production process can be run in batch sizes at scales that are sufficient to provide vaccine quantities either for interpandemic periods or even for pandemics. However, no vaccines have been licensed using this technology as yet.
 "
"