|
|
| (3 intermediate revisions not shown) |
| Line 174: |
Line 174: |
| | <br> | | <br> |
| | <br> | | <br> |
| - | <br>[[File:8-20110924.jpg|lefe|650px|caption]] | + | <br>[[File:8-20110924.jpg|lefe|550px|caption]][[File:Cats2.gif |right|404px|caption]] |
| | <br> | | <br> |
| | <br> | | <br> |
| Line 186: |
Line 186: |
| | <br> | | <br> |
| | <br> | | <br> |
| - | <br>[[File:11-20110927.jpg|lefe|650px|caption]] | + | <br>[[File:11-20110927.jpg|lefe|550px|caption]][[File:Cats3.jpg|right|404px|caption]] |
| | <br> | | <br> |
| | <br> | | <br> |
| Line 195: |
Line 195: |
| | <br> | | <br> |
| | <br>[[File:13-20110929.jpg|lefe|650px|caption]] | | <br>[[File:13-20110929.jpg|lefe|650px|caption]] |
| - |
| |
| - | <br><font color="#000000" size=3><b>2011.09.08-13</b></font>
| |
| | <br> | | <br> |
| - | <br><font color="#000000" size=2><b>Plasmid miniprep kit</b></font>
| |
| - | *PSB1C3 plasmid[[File:Cats.jpg|right|404px|caption]]
| |
| | <br> | | <br> |
| | <br> | | <br> |
| - | <br><font color="#000000" size=2><b>Raise Rhodobacter rubrum</b></font> | + | <br>[[File:14-20111001.jpg|lefe|650px|caption]] |
| - | :: 1.50ml LB+500μl CHLORAMPHENICOL
| + | |
| - | :: 2.37℃, overnight (14-16hrs)
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=3><b>2011.09.09</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Raise Gluconacetobacter hansenii</b></font>
| + | |
| - | :: 1.50ml LB+500μl CHLORAMPHENICOL
| + | |
| - | :: 2.37℃, overnight (14-16hrs)
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
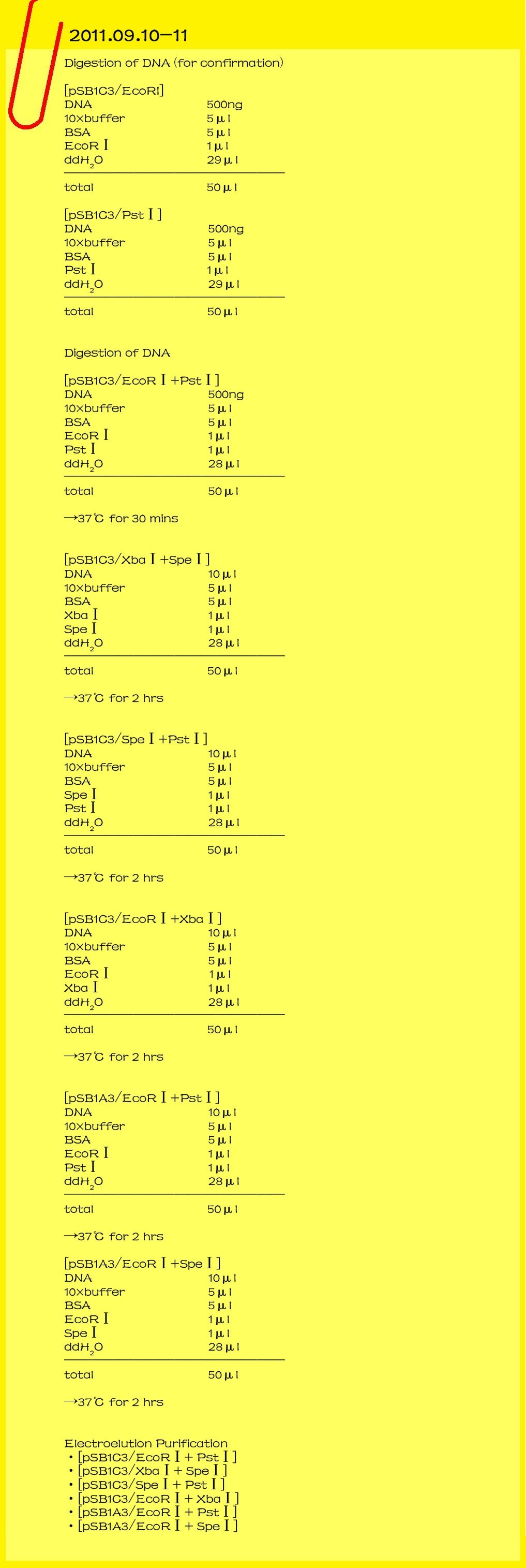
| - | <br><font color="#000000" size=3><b>2011.09.10-11</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Digestion check of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | [pSB1C3/EcoRI]
| + | |
| - | <br>DNA 500ng<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>ddH2O 29μl<br>
| + | |
| - | <br>_______________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1C3/PstⅠ]
| + | |
| - | <br>DNA 500ng<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>PstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 29μl<br>
| + | |
| - | <br>_________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Digestion of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | [pSB1C3/EcoRⅠ+PstⅠ]
| + | |
| - | <br>DNA 500ng<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>pstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>__________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 30 mins
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1C3/XbaⅠ+SpeⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>XbaⅠ 1μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 2 hrs
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1C3/SpeⅠ+PstⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>pstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 2 hrs
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1C3/EcoRⅠ+XbaⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>XbaⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 2 hrs
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1A3/EcoRⅠ+PstⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>PstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 2 hrs
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1A3/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 2 hrs
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>electroelution Purification</b></font>
| + | |
| - | <br>
| + | |
| - | *[pSB1C3/EcoRⅠ+PstⅠ]
| + | |
| - | *[pSB1C3/XbaⅠ+SpeⅠ]
| + | |
| - | *[pSB1C3/SpeⅠ+PstⅠ]
| + | |
| - | *[pSB1C3/EcoRⅠ+XbaⅠ]
| + | |
| - | *[pSB1A3/EcoRⅠ+PstⅠ]
| + | |
| - | *[pSB1A3/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
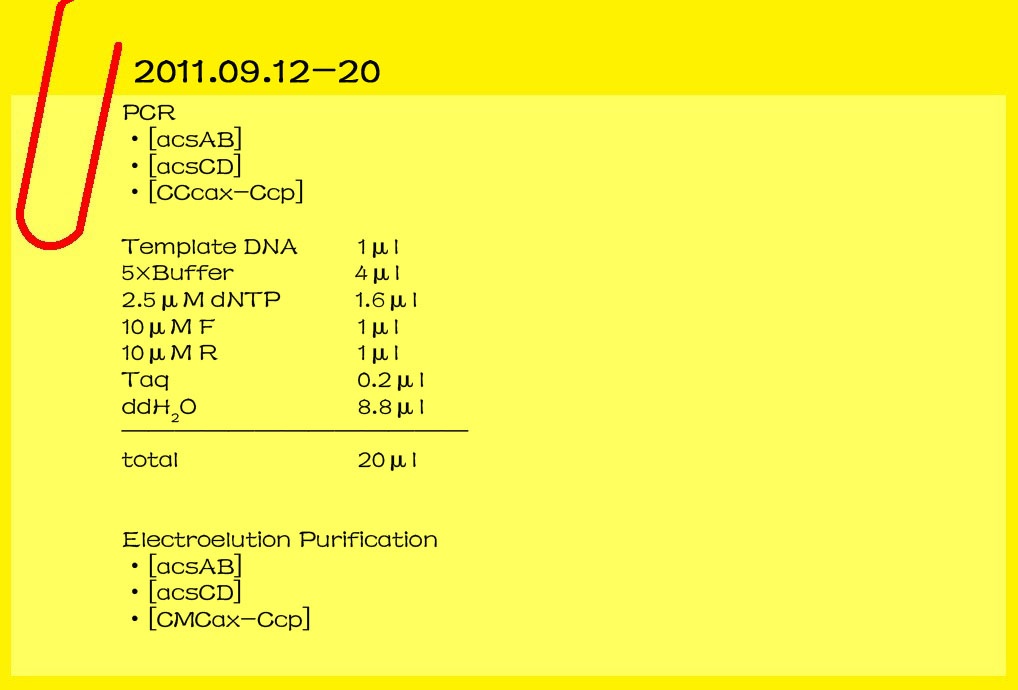
| - | <br><font color="#000000" size=3><b>2011.09.12-20</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>PCR</b></font>
| + | |
| - | [[File:Cats2.gif |right|404px|caption]] | + | |
| - | <br>
| + | |
| - | *[acsAB]
| + | |
| - | *[acsCD]
| + | |
| - | *[CCcax-Ccp]
| + | |
| - | <br>template DNA 1μl<br>
| + | |
| - | <br>5×Buffer 4μl<br>
| + | |
| - | <br>2.5μM dNTP 1.6μl<br>
| + | |
| - | <br>10μM F 1μl<br>
| + | |
| - | <br>10μM R 1μl<br>
| + | |
| - | <br>Taq 0.2μl<br>
| + | |
| - | <br>ddH2O 8.8μl<br>
| + | |
| - | <br>_______________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>electroelution Purification</b></font>
| + | |
| - | <br>
| + | |
| - | *[acsAB]
| + | |
| - | *[acsCD]
| + | |
| - | *[CMCax-Ccp]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=3><b>2011.09.21</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Digestion of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | [acsAB/ XbaⅠ+SpeⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>pstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [acsCD/XbaⅠ+SpeⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>XbaⅠ 1μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>__________________________________
| + | |
| - | <br>
| + | |
| - | total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [CMCax/SpeⅠ+AlwNⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>AlwNⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>__________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [Ccp/AlwNⅠ+PstⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>AlwNⅠ 1μl<br>
| + | |
| - | <br>PstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>__________________________________
| + | |
| - | <br>
| + | |
| - | total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>electroelution Purification</b></font>
| + | |
| - | <br>
| + | |
| - | *[acsAB/XbaⅠ+SpeⅠ]
| + | |
| - | *[acsCD/XbaⅠ+SpeⅠ]
| + | |
| - | *[CMCax/SpeⅠ+AlwNⅠ]
| + | |
| - | *[Ccp/AlwNⅠ+PstⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=3><b>2011.09.22</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Ligation of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | [pSB1C3-acsAB][[File:Cats3.jpg|right|404px|caption]]
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1A3-acsCD]
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>_________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1C3-acsCD]
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>_________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1C3-CMCax-Ccp]
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>_________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=3><b>2011.09.23</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>PCR</b></font>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [R0011 promoter]
| + | |
| - | <br>R0011 promoter 1μl<br>
| + | |
| - | <br>5×Buffer 4μl<br>
| + | |
| - | <br>2.5μM dNTP 1.6μl<br>
| + | |
| - | <br>Taq 0.2μl<br>
| + | |
| - | <br>ddH2O 13.2μl<br>
| + | |
| - | <br>_____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=3><b>electroelution Purification</b></font>
| + | |
| - | <br>
| + | |
| - | *[R0011 promoter]
| + | |
| - | <br><font color="#000000" size=2><b>Transformation of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | *[pSB1C3-acsAB]
| + | |
| - | :: Transform into E.coli
| + | |
| - | :: LB+CHLORAMPHENICOL
| + | |
| - | :: →37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | *[pSB1C3-acsCD]
| + | |
| - | :: Transform into E.coli
| + | |
| - | :: LB+CHLORAMPHENICOL
| + | |
| - | :: →37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | *[pSB1A3-acsCD]
| + | |
| - | :: Transform into E.coli
| + | |
| - | :: LB+Ampicillin
| + | |
| - | :: →37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | *[pSB1C3-CMCax-Ccp]
| + | |
| - | :: Transform into E.coli
| + | |
| - | :: LB+CHLORAMPHENICOL
| + | |
| - | :: →37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Digestion of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [R0011 promoter/EcoRⅠ+XbaⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>XbaⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>electroelution Purification</b></font>
| + | |
| - | <br>
| + | |
| - | *[R0011 promoter/EcoRⅠ+XbaⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=3><b>2011.09.24</b></font>
| + | |
| - | <br>
| + | |
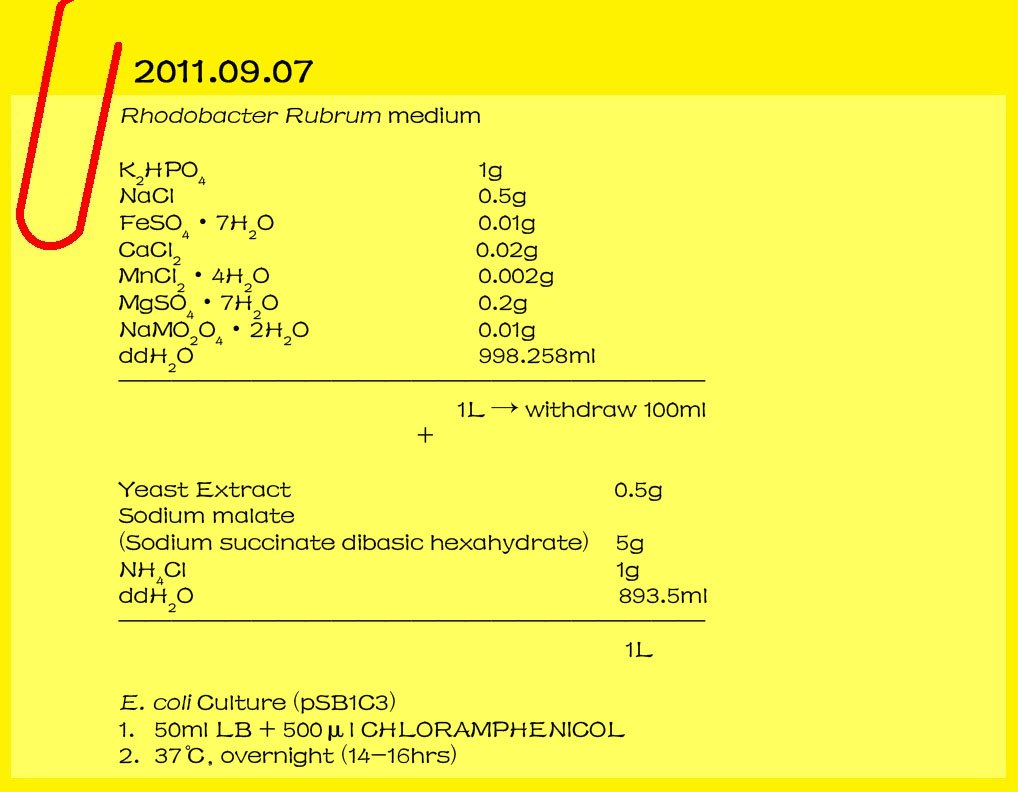
| - | <br><font color="#000000" size=2><b>Plasmid miniprep kit</b></font>
| + | |
| - | <br>
| + | |
| - | *[pSB1C3-acsAB]
| + | |
| - | *[pSB1C3-acsCD]
| + | |
| - | *[pSB1A3-acsCD]
| + | |
| - | *[pSB1C3-CMCax-Ccp]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Ligation of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | [pSB1C3-R0011]
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [R0011-acsAB]
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [R0011-acsCD]
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=3><b>2011.09.24</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Digestion of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | [R0011-acsAB/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [R0011-acsCD/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>electroelution Purification</b></font>
| + | |
| - | <br>
| + | |
| - | *[R0011-acsAB/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | *[R0011-acsCD/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
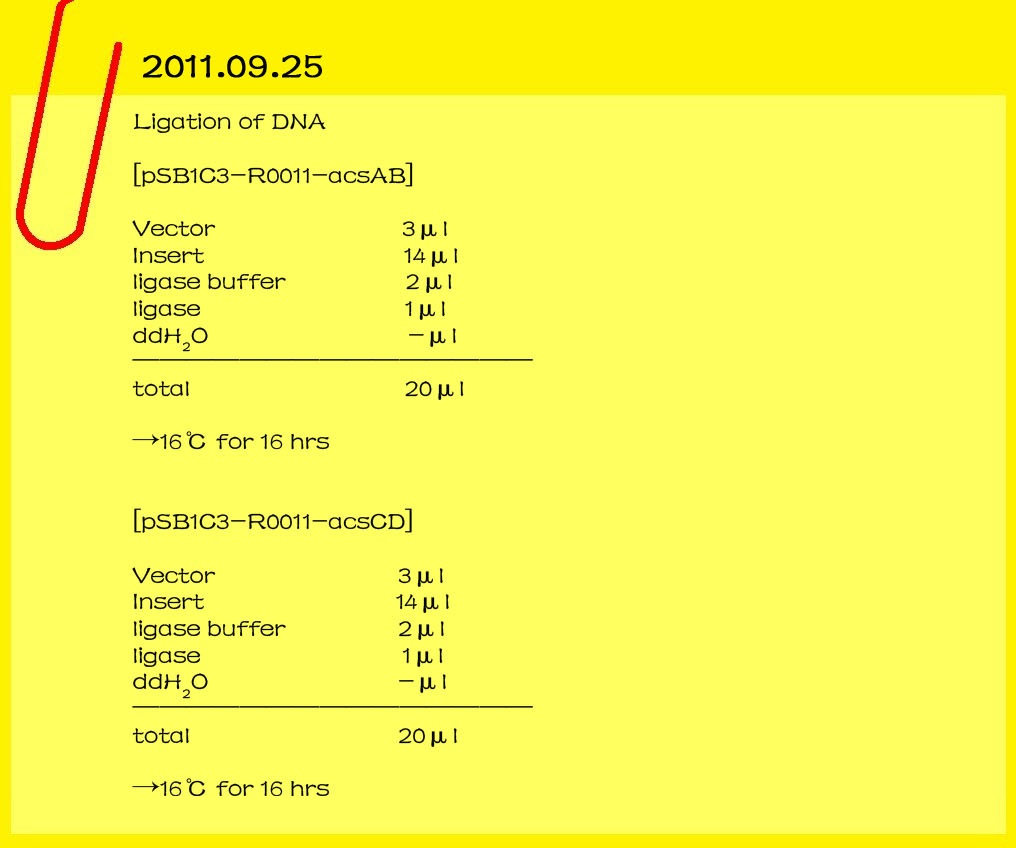
| - | <br><font color="#000000" size=3><b>2011.09.25</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Ligation of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | [pSB1C3-R0011-acsAB]
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1C3-R0011-acsCD]
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=3><b>2011.09.26</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Transformation of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | *[pSB1C3-R0011-acsAB]
| + | |
| - | :: Transform into E.coli
| + | |
| - | :: LB+CHLORAMPHENICOL
| + | |
| - | :: →37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | *[pSB1C3-R0011-acsCD]
| + | |
| - | :: Transform into E.coli
| + | |
| - | :: LB+CHLORAMPHENICOL
| + | |
| - | :: →37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Digestion of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | [pSB1C3-R0011-acsCD/SpeⅠ+PstⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>PstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>electroelution Purification</b></font>
| + | |
| - | *[pSB1C3-R0011-acsCD/SpeⅠ+PstⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=3><b>2011.09.27</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Plasmid miniprep kit</b></font>
| + | |
| - | <br>
| + | |
| - | *[pSB1C3-R0011-acsAB]
| + | |
| - | *[pSB1C3-R0011-acsCD]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Ligation of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | [pSB1C3-R0011-acsCD-CMCax-Ccp]
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Digestion of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1C3-R0011-acsCD-CMCax-Ccp/PstⅠ+EcoRⅠ]
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>PstⅠ 1μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>electroelution Purification</b></font>
| + | |
| - | *[pSB1C3-R0011-acsCD-CMCax-Ccp/PstⅠ+EcoRⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
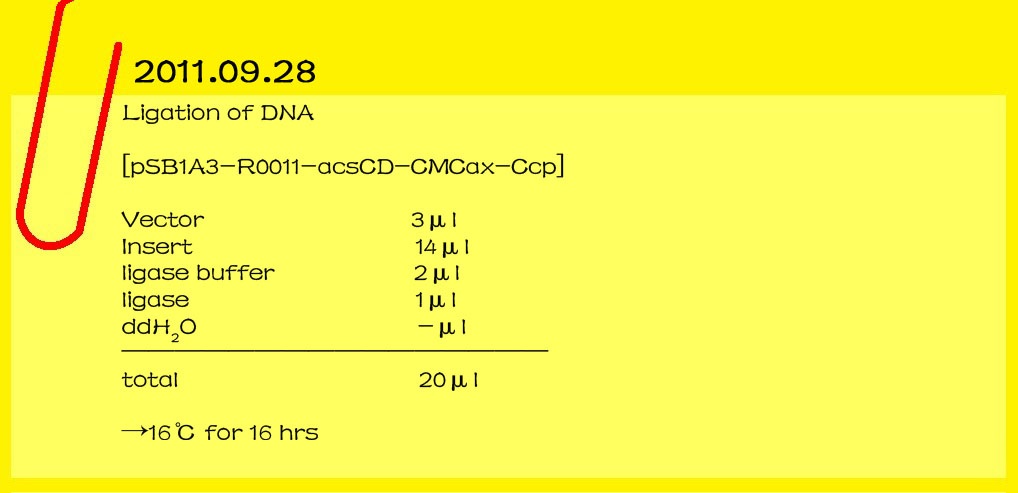
| - | <br><font color="#000000" size=3><b>2011.09.28</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Ligation of DNA</b></font>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | [pSB1A3-R0011-acsCD-CMCax-Ccp]
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=3><b>2011.09.29</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>Transformation of DNA</b></font>
| + | |
| - | <br>
| + | |
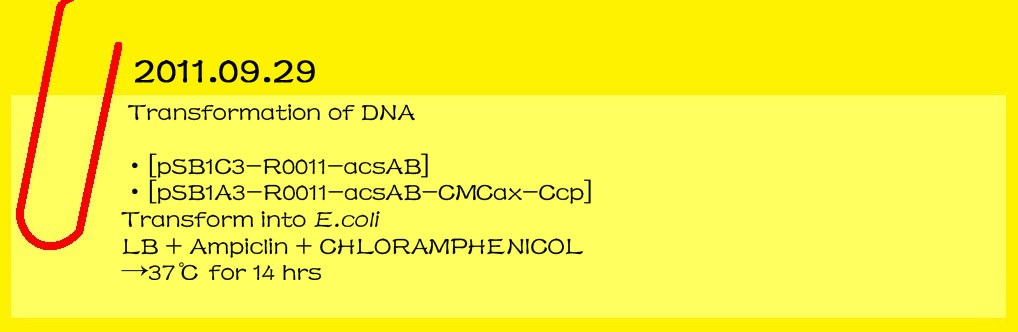
| - | *[pSB1C3-R0011-acsAB]
| + | |
| - | *[pSB1A3-R0011-acsAB-CMCax-Ccp]
| + | |
| - | :: Transform into E.coli
| + | |
| - | :: LB+Ampiclin+CHLORAMPHENICOL
| + | |
| - | :: →37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=3><b>2011.10.01</b></font>
| + | |
| - | <br>
| + | |
| - | <br><font color="#000000" size=2><b>modelling test</b></font>
| + | |
| | <br> | | <br> |
| | <br> | | <br> |
| | <br> | | <br> |





 "
"