|
|
| (27 intermediate revisions not shown) |
| Line 26: |
Line 26: |
| | </html> | | </html> |
| | <font color="#228B22" size=6>Photopaper</font> | | <font color="#228B22" size=6>Photopaper</font> |
| - | <br><br><font color="#000080" size=4>Meeting Notes</font> | + | <br><br><font color="#000080" size=4>Meeting Minutes</font> |
| | <Hr Align="left" width="100%" size=2> | | <Hr Align="left" width="100%" size=2> |
| | <font color="#000000" size=3><b>2011.02.24</b></font> | | <font color="#000000" size=3><b>2011.02.24</b></font> |
| Line 145: |
Line 145: |
| | <Hr Align="left" width="100%" size=3> | | <Hr Align="left" width="100%" size=3> |
| | | | |
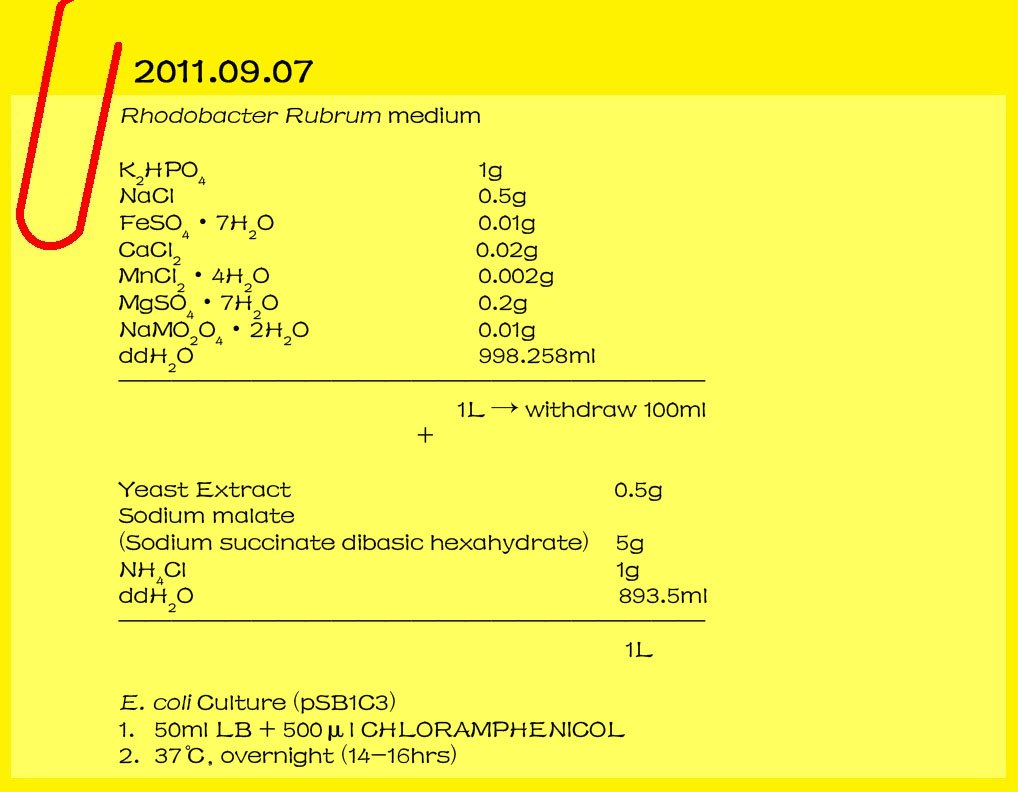
| - | <font size=3>2011.09.07</font>
| |
| - | <br>[[File:Catt.gif|right|250px|caption]]
| |
| - | Rhodobacter rudrum medium
| |
| - | <br>
| |
| - | <br>K2HPO4 1g<br>
| |
| - | <br>NaCl 0.5g<br>
| |
| - | <br>FeSO4.7H2O 0.01g<br>
| |
| - | <br>CaCl2 0.02g<br>
| |
| - | <br>MnCl2.4H2O 0.002g<br>
| |
| - | <br>MgSO4.7H2O 0.2g<br>
| |
| - | <br>NaMO2O4.2H2O 0.01g<br>
| |
| - | <br>ddH2O 998.258ml<br>
| |
| - | <br>________________________________________
| |
| - | <br> 1L →take100ml<br>
| |
| - |
| |
| - | +
| |
| | | | |
| - | <br>Yeast Extrat 0.5g<br> | + | <br>[[File:1-20110907.jpg|lefe|650px|caption]][[File:Catt.gif|right|250px|caption]] |
| - | <br>Sodium malate
| + | |
| - | <br>(Sodium succinate dibasic hexohydrate) 5g<br>
| + | |
| - | <br>NH4Cl 1g<br>
| + | |
| - | <br>ddH2O 893.5ml<br>
| + | |
| - | <br>_________________________________________________
| + | |
| - | <br> 1L<br>
| + | |
| | <br> | | <br> |
| | <br> | | <br> |
| - | <br><font color="#000000" size=2><b>Raise E. coli(PSB1C3)</B></font>
| |
| | <br> | | <br> |
| - | <br>1.50ml LB+500μl CHLORAMPHENICOL | + | <br>[[File:2-20110908-13.jpg|lefe|650px|caption]] |
| - | <br>2.37℃, overnight (14-16hrs)
| + | |
| | <br> | | <br> |
| | <br> | | <br> |
| | <br> | | <br> |
| - | ==='''2011.09.08-13'''===
| + | <br>[[File:3-20110910-11.jpg|lefe|650px|caption]] |
| | <br> | | <br> |
| - | '''Plasmid miniprep kit'''
| |
| - | <br>PSB1C3 plasmid[[File:Cats.jpg|right|404px|caption]]
| |
| | <br> | | <br> |
| | <br> | | <br> |
| - | '''Raise Rhodobacter rubrum'''
| + | <br>[[File:4-20110912-20.jpg|lefe|650px|caption]] |
| | <br> | | <br> |
| - | <br>1.50ml LB+500μl CHLORAMPHENICOL
| |
| - | <br>2.37℃, overnight (14-16hrs)
| |
| | <br> | | <br> |
| | <br> | | <br> |
| | + | <br>[[File:5-20110921.jpg|lefe|550px|caption]][[File:Cats.jpg|right|404px|caption]] |
| | <br> | | <br> |
| - | ==='''2011.09.09'''===
| |
| | <br> | | <br> |
| - | '''Raise Gluconacetobacter hansenii'''
| |
| | <br> | | <br> |
| - | <br>1.50ml LB+500μl CHLORAMPHENICOL | + | <br>[[File:6-20110922.jpg|lefe|650px|caption]] |
| - | <br>2.37℃, overnight (14-16hrs)
| + | |
| | <br> | | <br> |
| | <br> | | <br> |
| | <br> | | <br> |
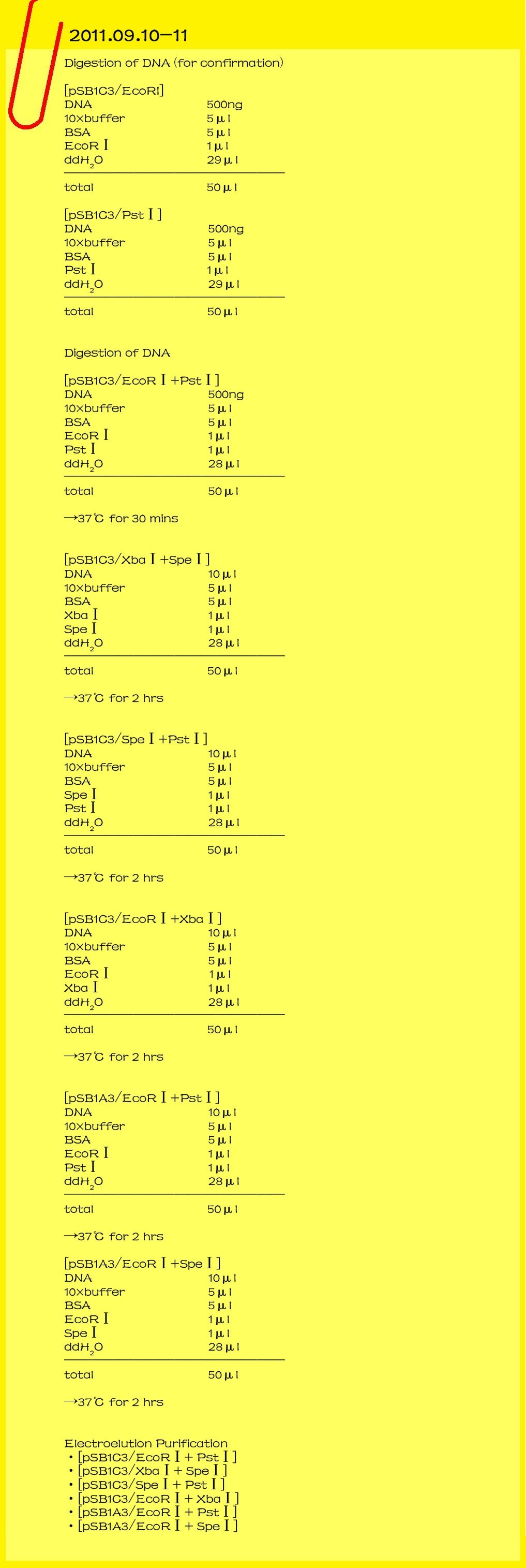
| - | ==='''2011.09.10-11'''===
| + | <br>[[File:7-20110923.jpg|lefe|650px|caption]] |
| | <br> | | <br> |
| - | '''Digestion check of DNA'''
| |
| | <br> | | <br> |
| - | <br>[pSB1C3/EcoRI]
| |
| | <br> | | <br> |
| - | <br>DNA 500ng<br> | + | <br>[[File:8-20110924.jpg|lefe|550px|caption]][[File:Cats2.gif |right|404px|caption]] |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>ddH2O 29μl<br>
| + | |
| - | <br>_______________________________
| + | |
| | <br> | | <br> |
| - | <br>total 50μl<br>
| |
| | <br> | | <br> |
| | <br> | | <br> |
| - | <br>[pSB1C3/PstⅠ] | + | <br>[[File:9-20110925.jpg|lefe|650px|caption]] |
| | <br> | | <br> |
| - | <br>DNA 500ng<br>
| |
| - | <br>10×buffer 5μl<br>
| |
| - | <br>BSA 5μl<br>
| |
| - | <br>PstⅠ 1μl<br>
| |
| - | <br>ddH2O 29μl<br>
| |
| - | <br>_________________________________
| |
| | <br> | | <br> |
| - | <br>total 50μl<br>
| |
| | <br> | | <br> |
| | + | <br>[[File:10-20110926.jpg|lefe|650px|caption]] |
| | <br> | | <br> |
| - | '''Digestion of DNA'''
| |
| | <br> | | <br> |
| - | <br>[pSB1C3/EcoRⅠ+PstⅠ]
| |
| | <br> | | <br> |
| - | <br>DNA 500ng<br>
| + | <br>[[File:11-20110927.jpg|lefe|550px|caption]][[File:Cats3.jpg|right|404px|caption]] |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>pstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>__________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 30 mins
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3/XbaⅠ+SpeⅠ] | + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>XbaⅠ 1μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 2 hrs
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3/SpeⅠ+PstⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>pstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 2 hrs
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3/EcoRⅠ+XbaⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>XbaⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 2 hrs
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[pSB1A3/EcoRⅠ+PstⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>PstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 2 hrs
| + | |
| - | <br>
| + | |
| - | <br>[pSB1A3/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 2 hrs
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''electroelution Purification'''
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3/EcoRⅠ+PstⅠ]
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3/XbaⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3/SpeⅠ+PstⅠ]
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3/EcoRⅠ+XbaⅠ]
| + | |
| - | <br>
| + | |
| - | <br>[pSB1A3/EcoRⅠ+PstⅠ]
| + | |
| - | <br>
| + | |
| - | <br>[pSB1A3/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
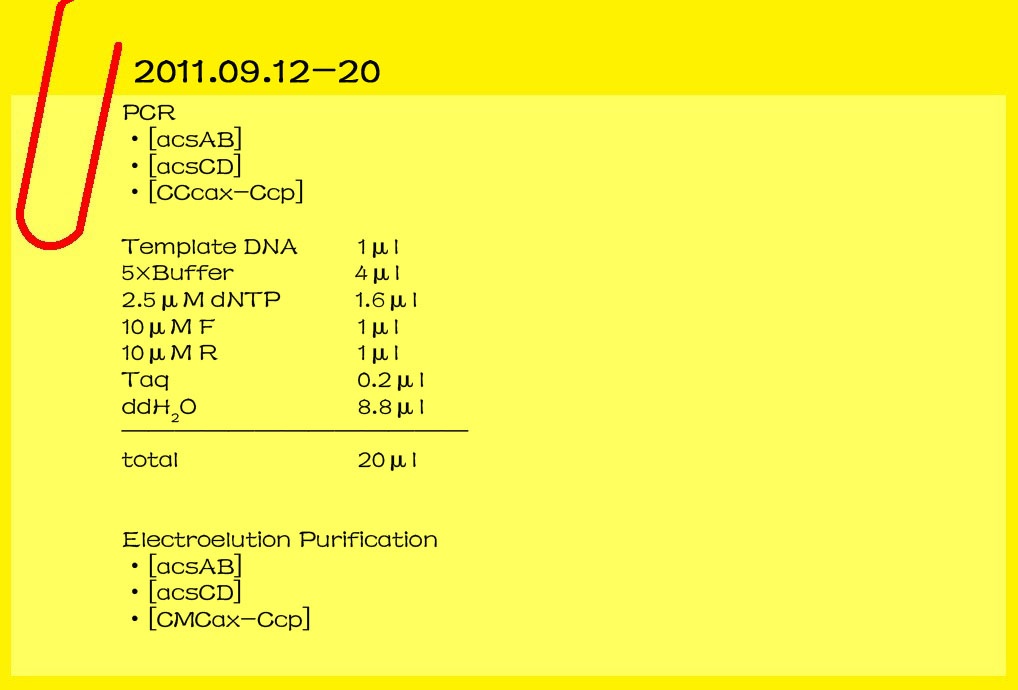
| - | ==='''2011.09.12-20'''===
| + | |
| - | <br>
| + | |
| - | '''PCR'''
| + | |
| - | [[File:Cats2.gif |right|404px|caption]] | + | |
| - | <br>[acsAB]
| + | |
| - | <br>
| + | |
| - | <br>[acsCD]
| + | |
| - | <br>
| + | |
| - | <br>[CCcax-Ccp]
| + | |
| - | <br>
| + | |
| - | <br>template DNA 1μl<br>
| + | |
| - | <br>5×Buffer 4μl<br>
| + | |
| - | <br>2.5μM dNTP 1.6μl<br>
| + | |
| - | <br>10μM F 1μl<br>
| + | |
| - | <br>10μM R 1μl<br>
| + | |
| - | <br>Taq 0.2μl<br>
| + | |
| - | <br>ddH2O 8.8μl<br>
| + | |
| - | <br>_______________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''electroelution Purification'''
| + | |
| - | <br>
| + | |
| - | <br>[acsAB]
| + | |
| - | <br>
| + | |
| - | <br>[acsCD]
| + | |
| - | <br>
| + | |
| - | <br>[CMCax-Ccp]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ==='''2011.09.21'''===
| + | |
| - | <br>
| + | |
| - | '''Digestion of DNA'''
| + | |
| - | <br>
| + | |
| - | <br>[acsAB/ XbaⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>pstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[acsCD/XbaⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>XbaⅠ 1μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>__________________________________
| + | |
| - | <br>
| + | |
| - | total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[CMCax/SpeⅠ+AlwNⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>AlwNⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>__________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[Ccp/AlwNⅠ+PstⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>AlwNⅠ 1μl<br>
| + | |
| - | <br>PstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>__________________________________
| + | |
| - | <br>
| + | |
| - | total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''electroelution Purification'''
| + | |
| - | <br>
| + | |
| - | <br>[acsAB/ XbaⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>[acsCD/XbaⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>[CMCax/SpeⅠ+AlwNⅠ]
| + | |
| - | <br>
| + | |
| - | <br>[Ccp/AlwNⅠ+PstⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ==='''2011.09.22'''===
| + | |
| - | <br>
| + | |
| - | '''Ligation of DNA'''
| + | |
| - | <br>[pSB1C3-acsAB][[File:Cats3.jpg|right|404px|caption]]
| + | |
| - | <br>
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[pSB1A3-acsCD]
| + | |
| - | <br>
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>_________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-acsCD]
| + | |
| - | <br>
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>_________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-CMCax-Ccp]
| + | |
| - | <br>
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>_________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ==='''2011.09.23'''===
| + | |
| - | <br>
| + | |
| - | '''PCR'''
| + | |
| - | <br>
| + | |
| - | <br>[R0011 promoter]
| + | |
| - | <br>
| + | |
| - | <br>R0011 promoter 1μl<br>
| + | |
| - | <br>5×Buffer 4μl<br>
| + | |
| - | <br>2.5μM dNTP 1.6μl<br>
| + | |
| - | <br>Taq 0.2μl<br>
| + | |
| - | <br>ddH2O 13.2μl<br>
| + | |
| - | <br>_____________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''electroelution Purification'''
| + | |
| - | <br>
| + | |
| - | <br>[R0011 promoter]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''Transformation of DNA'''
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-acsAB]
| + | |
| - | <br>
| + | |
| - | <br>Transform into E.coli
| + | |
| - | <br>LB+CHLORAMPHENICOL
| + | |
| - | <br>→37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-acsCD]
| + | |
| - | <br>
| + | |
| - | <br>Transform into E.coli
| + | |
| - | <br>LB+CHLORAMPHENICOL
| + | |
| - | <br>→37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>[pSB1A3-acsCD]
| + | |
| - | <br>
| + | |
| - | <br>Transform into E.coli
| + | |
| - | <br>LB+Ampicillin
| + | |
| - | <br>→37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-CMCax-Ccp]
| + | |
| - | <br>
| + | |
| - | <br>Transform into E.coli
| + | |
| - | <br>LB+CHLORAMPHENICOL
| + | |
| - | <br>→37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''Digestion of DNA'''
| + | |
| - | <br>
| + | |
| - | <br>[R0011 promoter/EcoRⅠ+XbaⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>XbaⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | | + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''electroelution Purification'''
| + | |
| - | <br>
| + | |
| - | <br>[R0011 promoter/EcoRⅠ+XbaⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ==='''2011.09.24'''===
| + | |
| - | <br>
| + | |
| - | '''Ligation of DNA'''
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-R0011]
| + | |
| - | <br>
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[R0011-acsAB]
| + | |
| - | <br>
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>[R0011-acsCD]
| + | |
| - | <br>
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ==='''2011.09.24'''===
| + | |
| - | <br>
| + | |
| - | '''Digestion of DNA'''
| + | |
| - | <br>
| + | |
| - | <br>[R0011-acsAB/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | | + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>[R0011-acsCD/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | <br>
| + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''electroelution Purification'''
| + | |
| - | <br>
| + | |
| - | <br>[R0011-acsAB/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>[R0011-acsCD/EcoRⅠ+SpeⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
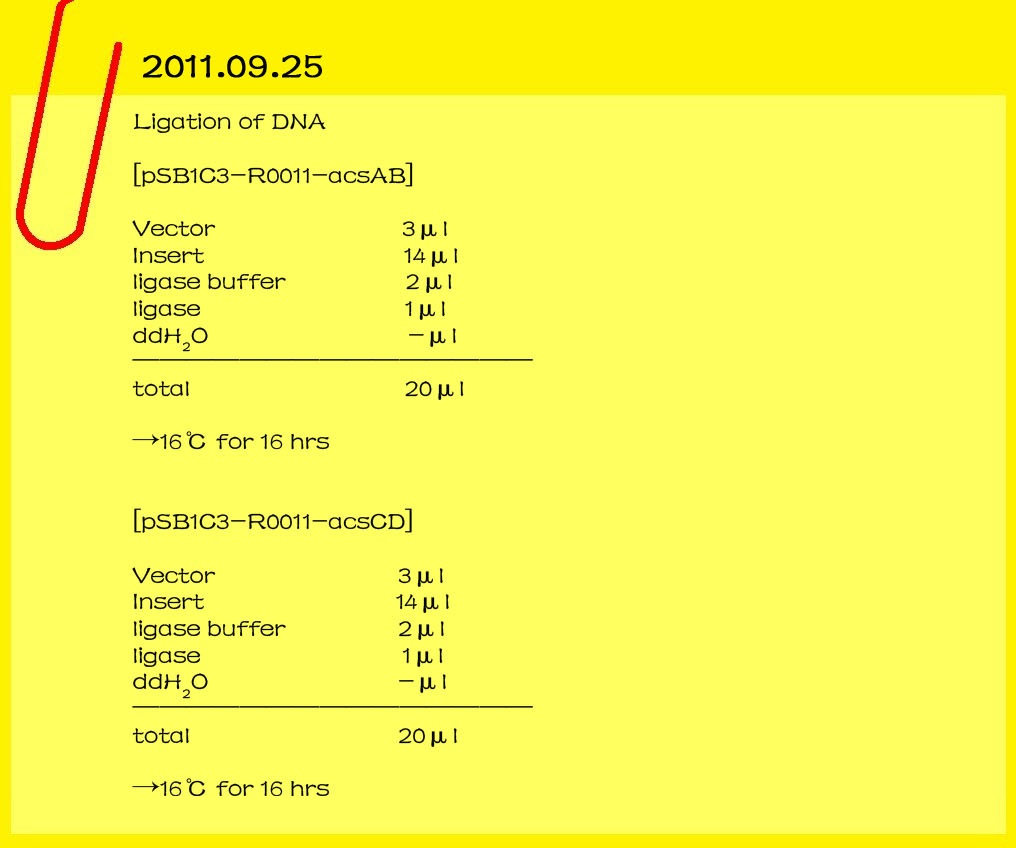
| - | ==='''2011.09.25'''===
| + | |
| - | <br>
| + | |
| - | '''Ligation of DNA'''
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-R0011-acsAB]
| + | |
| - | <br>
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-R0011-acsCD]
| + | |
| - | <br>
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ==='''2011.09.26'''===
| + | |
| - | <br>
| + | |
| - | '''Transformation of DNA'''
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-R0011-acsAB]
| + | |
| - | <br>
| + | |
| - | <br>Transform into E.coli
| + | |
| - | <br>LB+CHLORAMPHENICOL
| + | |
| - | <br>→37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-R0011-acsCD]
| + | |
| - | <br>
| + | |
| - | <br>Transform into E.coli
| + | |
| - | <br>LB+CHLORAMPHENICOL
| + | |
| - | <br>→37℃ for 14 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''Digestion of DNA'''
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-R0011-acsCD/SpeⅠ+PstⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>SpeⅠ 1μl<br>
| + | |
| - | <br>PstⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | | + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''electroelution Purification'''
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-R0011-acsCD/SpeⅠ+PstⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
| - | ==='''2011.09.27'''===
| + | |
| - | <br>
| + | |
| - | '''Ligation of DNA'''
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-R0011-acsCD-CMCax-Ccp]
| + | |
| - | <br>
| + | |
| - | <br>Vector 3μl<br>
| + | |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| - | <br>
| + | |
| - | <br>total 20μl<br>
| + | |
| - | <br>→16℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''Digestion of DNA'''
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-R0011-acsCD-CMCax-Ccp/PstⅠ+EcoRⅠ]
| + | |
| - | <br>
| + | |
| - | <br>DNA 10μl<br>
| + | |
| - | <br>10×buffer 5μl<br>
| + | |
| - | <br>BSA 5μl<br>
| + | |
| - | <br>PstⅠ 1μl<br>
| + | |
| - | <br>EcoRⅠ 1μl<br>
| + | |
| - | <br>ddH2O 28μl<br>
| + | |
| - | <br>____________________________
| + | |
| - | | + | |
| - | <br>total 50μl<br>
| + | |
| - | <br>→37℃ for 16 hr
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | '''electroelution Purification'''
| + | |
| - | <br>
| + | |
| - | <br>[pSB1C3-R0011-acsCD-CMCax-Ccp/PstⅠ+EcoRⅠ]
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | <br>
| + | |
| - | | + | |
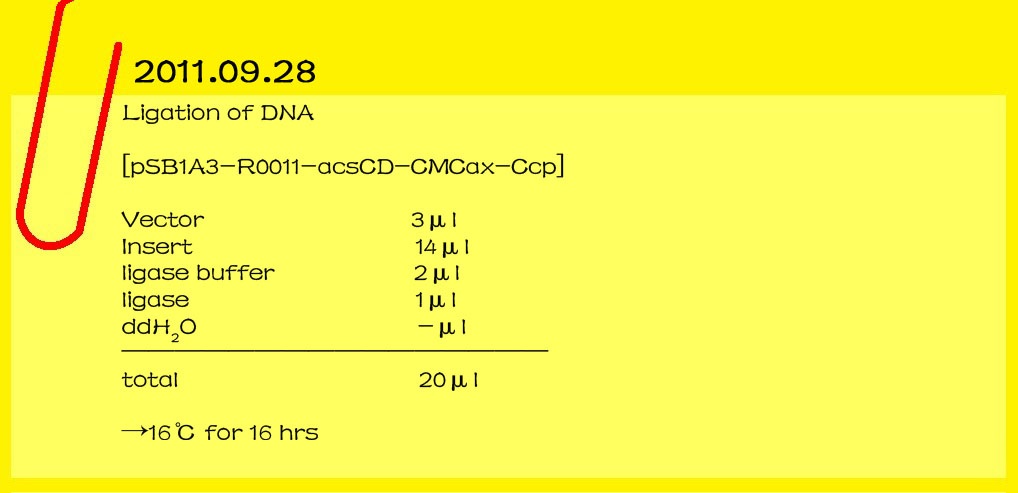
| - | ==='''2011.09.28'''===
| + | |
| | <br> | | <br> |
| - | '''Ligation of DNA'''
| |
| | <br> | | <br> |
| - | <br>[pSB1A3-R0011-acsCD-CMCax-Ccp]
| |
| | <br> | | <br> |
| - | <br>Vector 3μl<br> | + | <br>[[File:12-20110928.jpg|lefe|650px|caption]] |
| - | <br>Insert 14μl<br>
| + | |
| - | <br>ligase buffer 2μl<br>
| + | |
| - | <br>ligase 1μl<br>
| + | |
| - | <br>ddH2O -μl<br>
| + | |
| - | <br>________________________________
| + | |
| | <br> | | <br> |
| - | <br>total 20μl<br>
| |
| - | <br>→16℃ for 16 hr
| |
| | <br> | | <br> |
| | <br> | | <br> |
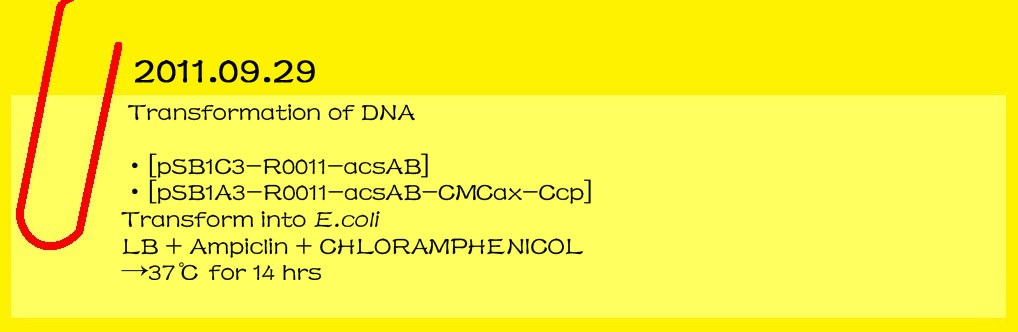
| - | ==='''2011.09.29'''===
| + | <br>[[File:13-20110929.jpg|lefe|650px|caption]] |
| | <br> | | <br> |
| - | '''Transformation of DNA'''
| |
| | <br> | | <br> |
| - | <br>[pSB1C3-R0011-acsAB]
| |
| - | <br>[pSB1A3-R0011-acsAB-CMCax-Ccp]
| |
| | <br> | | <br> |
| - | <br>Transform into E.coli | + | <br>[[File:14-20111001.jpg|lefe|650px|caption]] |
| - | <br>LB+Ampiclin+CHLORAMPHENICOL
| + | |
| - | <br>→37℃ for 14 hr
| + | |
| | <br> | | <br> |
| | <br> | | <br> |
| | <br> | | <br> |





 "
"