Team:TU Munich/lab/notebook/part3
From 2011.igem.org
22-08-2011
Daily Work (Alex, Simon)
Digestion:
-part "T7" (date: 17.6., concentration = 190 ng/µl) was digested using X and P. At first we thought that this is a T7 polymerase part (the tube wasn't properly labeled!!!), but later we found out that this is really a T7 promoter.
- part "RBS minip" (date: 22.6., concentration = 47.5 ng/µl) was digested using S and P.
- part "K238013 cm" (date: 29.6., concentration = 233 ng/µl) was digested using S and P.
Analytic & preparative Gel:
The samples from the restriction digests (see above) were separated using a 1% Agarosegel. Expectations: T7prom cut with X,P should yield a band at approximately 3.5 kbp. Rbs cut with S,P should yield a band at approximately 2 kbp. K238013 in pSB3C5 should yield a band at approximately 2.8 kbp. In pSB1A2 it should be 2 kbp.
The original photography cannot be shown, as the file seems to be corrupt. The bands all fit, except for K238013. The content of this plasmid seems unclear, as the length in base pairs is much higher than expected. For the upcoming ligation, another K238013 should be used.
Inoculation: 5 ml LB (Kan) were inoculated with two further colonies from the transformations of the reporter plasmid (part2b) into BL21 from DATUM. The cultures were grown over night.
LB S-Gal Paltes: S-Gal plates were produced, using 500 ml ddH2O, 12.5 mg LB, 300 mg S-Gal and 460 mg ferric ammonium sulfate. After autoclavation, the solutions were tempered to about 58 °C and Kan or Cm was added. The solutions were spilled into plates and are kept in the fridge.
Results
Sequencing: Some of the sequenced data arrived. Part 8a (from 14-07-2011; T7Pol & rbs) does NOT contain the desired rbs. The T7Pol seems to be inserted alright. For the rest of the data, GATC awaits the arrival of the necessary primers.
23-08-2011
Daily Work (Alex, Simon, Bea, Thorsten)
Digestion: We digested blue light K from 29-06-2011 (S,P), K238013 in unknown plasmid (S,P), lacZ (X,P) and pSB3C5 (S,P). After digestion, the samples were applied onto a 1 % gel. The lanes are as follows (from left to right): 2log DNA ladder (3 ul); lacZ (20 ul); blue light K (20 ul); K238013 (20 ul); pSB3C5 (20 ul); K228000 (20 ul); lacZ (20 ul); blue light K (20 ul); K238013 (20 ul); pSB3C5 (20 ul). LacZ did not show any bands and blue light K yielded a band at an unexpected heigth, but the rest of the bands are as expected. The following parts were extracted and frozen: Blue light K at ca. 3 kbp; K238013 at ca. 2.2 kbp; pSB3C5 at ca. 2.7 kbp; T7pol at ca. 2.7 kbp and the plasmid of T7pol (pSB1A2) at ca. 2 kbp. They will be used for ligation tomorrow.
Induction of reporter plasmid: The OD_600 was measured from the LB (Kan) cultures inoculated with the reporter plasmid (part2b) in BL21, which were incubated over night. At an OD_600 of about 0.7, the cultures were split and one half of the cultures were induced with IPTG (c_final = 1mM). After a few hours, the cultures were plated onto S-Gal plates with Kann and incubated over night.
Transformation: Blue light K from 29-06-2011 was transformed into DH5alpha cells and plated onto LB (Cm) plates, which were incubated over night.
Test of K322127: 5 ml LB with Cm were inoculated each with DH5alpha transformed with K322127. The cultures are incubated over night during exposition to a LED with approximately 630 nm.
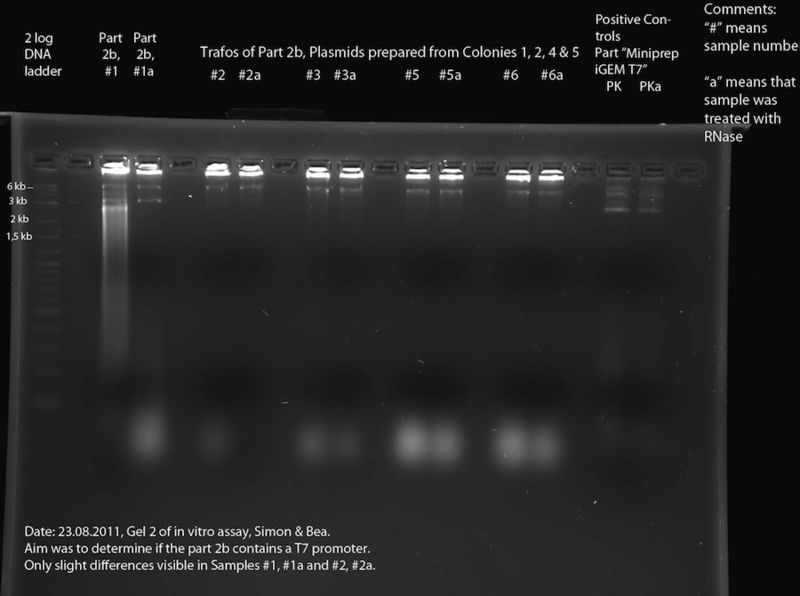
In Vitro Assay (Bea, Simon):
To assess the reporter plasmid ("Part 2b"). According to the DNA Sequence (see meetings 22.08. and 15.8) the part contains lacZ, but the sequence data is bad in the region of the T7 promotor. To find out whether the T7 promotor is present, we performed an in vitro assay. At first, the DNA samples of interest were transcribed using a T7 polymerase (for exact composition of reaction mixture see below), incubation time: 2h at 37 °C. After this, each sample was split in two halves. One of these each of these halves was then incubated at 37 °C for 30 minutes in presence of RNase. Finally, the different samples were separated in a 2% Agarose gel. The RNase should destroy the RNA produced by the T7 polymerase. Thus if two corresponding lines of the gel are compared, bands that only appear in one of the two lines are caused by RNA. This means that the respective DNA construct contained a T7 promoter. For exact protocol also see Methods.
The following DNA samples were transcribed in vitro using a T7 polymerase: Sample Number - Part Name - Date - concentration (ng/ul)
- 1 - 2b - 13.07.2011 - 215
- 2 - Trafo 2b Nr. 1 - 17.08.2011 - 106
- 3 - Trafo 2b Nr. 2 - 17.08.2011 - 121
- 4 - mistake - was thrown away
- 5 - Trafo 2b Nr. 4 - 17.08.2011 - 130
- 6 - Trafo 2b Nr. 5 - 17.08.2011 - 118
- Positive Control (PK) - Miniprep iGEM T7 - 31.05.11 - 58
The size of the construct in samples 1 - 6 is approx. 6.6 kb, the size of the construct of the positive Control is approx. 3.4 kb. Thus, the number of plasmids per microliter is approximately the same in all samples (only half the amount of sample 1 was used).
The reaction mixture contained: 2 µl buffer (40 mM TrisHCl, 40 mM MgCl2), 2 µl DTT (10 mM), 2 µl T7 Polymerase (50 u /µl), 10 µl DNA (only 5 µl in Sample 1), 1µl rNTPs (4 mM), fill up to 20 µl with ddH2O.
After incubation (2h at 37 °C), 10 µl of each sample was taken and mixed with 1 µl of RNase and incubated at 37 °C for 30 minutes. After this, all 12 samples were separated using a 2 % Agarose Gel (2log DNA ladder). Gel electrophoresis, 100 V, 400 mA, ca. 1:30 h.
Results
Transformations: The transformation of K238013 into DH5alpha cells on LB with Cm from yesterday was successful. However, all the colonies were pink (part not inserted!?). The T7promotor grew on Amp and Kan. From the last days: The transformation of B0015 and K238013 yielded pink bands (part not inserted!?).
In Vitro Assay
Samples #2 through #6a look very much alike, with the only difference being the "blurry blobs" at the bottom of the gel (not visible in #2a). We assume that these "blobs" are caused by RNA fragments, which leads us to the conclusion that RNase was present in all of these samples. The reason for this might be the fact that these samples were prepared from cells without performing an ethanol precipitation and thus, RNase from the cells might contaminate the samples. Looking at the samples #5 and #5a as well as #6 and #6a, for example, there is a band at about 6 kb. This must be DNA. This and the fact that there is digested RNA visible at the bottom leads us to the conclusion, that the band at 6 kb is a plasmid containing a T7 promoter (--> accordingly, this should be the correct plasmid containing T7 promoter and lacZ). To verify this, a second in vitro assay should be performed after inhibition of RNase in these samples. Comparison of #1 and #1a shows that part 2b must contain a T7 promoter, because of the blurred signal in #1 which is missing in #1b. This must be RNA which was digested by the RNase present in #1b. In both #1 and #1a there are bands at approximately 6 kb and 3 kb (6 kb could be the backbone containing T7 promoter and lacZ, 3 kb could be backbone containing only the t7 promoter). This DNA might be two different plasmids. This explains why the sequencing results are bad. At least one of these plasmids contains a T7 promoter, but it is unclear which one.
24-08-2011
Daily Work (Alex, Simon, Bea, Thorsten)
Ligations and transformations (K238013/lacZ; rbs/T7Pol; T7Prom/lacZ)
Ligation and transformation: Three ligations in total were conducted. K238013 (S,P) was quick-ligated with lacZ (X,P), rbs (S,P) with T7Pol (X,P) and T7Prom (S,P) with lacZ (X,P). The ligations were transformed into DH5alpha. Additionally, K238013 (from23-08-2011) was transformed into DH5alpha. The ligation of K238013 with lacZ is plated onto Amp and Cm, rbs with T7pol is plated onto Amp, T7Prom with lacZ onto Kan and K238013 is plated onto Amp and Cm.
Expression test of K322127
The two cultures of K322127 in DH5alpha incubated over night with red light (ca. 630 nm) were split. One half was further incubated with red light, while the other half was incubated with sun light and light from a table-lamp over night.
Test of negative controls on S-Gal: T7prom (Kan) transformed into DH5alpha and K322127 (Cm) transformed into BL21 were inoculated onto S-Gal plates with the respective antibiotics. The are incubated over night, and should not yield black colonies, as they both should not be able to express functional lacZ.
Results
Expression test of reporter plasmid: The cultures with ligation of T7prom and lacZ transformed into BL21 and induced with IPTG showed black colonies on the S-Gal plates. However, the cultures, which were NOT induced also showed black colonies of the same intensity. At the current point, we cannot sufficiently explain this.
Red light sensor assembly (Bea and Thorsten)
aim:
Ligation of red light sensor didn't work, yet. Therefore we ligated PCR product of BBa_K322127(X and P cut) into pSB3C5(S and P cut) to verify correct sites. Subsequent amplification and recutting of this part should lead to correct ligation with SupD.
In parallel, PCR was performed again in case of failed ligations with existent PCR-products.
Materials:
digest:
pSB3C5: S and P cut by Alex on 23.08.11, CAM resistance, 2,7 kbp vector backbone
PCR product of BBa_K322127 with primers BBa_K568000, derived from Biomers, sequence see below, 3,8 kbp (Florian 6.7.11 labelled with "K322127 PCR product aus gel ausgeschnitten")
pcr:
Template: BBa_K322127 ("Edinburgh part") conc: 150 ng/µl
primers (synthesized by biomers, 29.6.11, stock: 100µM):
- BBa_K568000_fwd: tatatctatatcgaattcgcggccgcttctagagtttacggctagctcagtcctaggta (59bp)
- BBa_K568000_rev: cgtgccggcggctgcagcggccgctactagtaagtccattctccccaaaaatg (53bp)
Procedure
digest:
10 µl PCR Product was cut with 1 µl XbaI and 1 µl PstI in 5 µl NEB4-buffer (total volume 50 µl) for 2h at 37°C with subsequent heat inactivation at 80°C for 20 minutes. Cut PCR-product was loaded on a 1% Agarose Gel in TAE buffer and run at 120 V for 1 h.
PCR:
amplification with Taq PCR Kit (NEB) according to Florian at 5.7.11. No water control was run due to lacking remaining buffer volume.
- 5 µl 10x standard buffer
- 1 µl dNTP's
- 0,25 µl Tag DNA polymerase
- 0,5 µl primers, respectively
- 0,4 µl Template DNA
- add up to 50 µl with sterile water
PCR-program:
- see 5.7.11
Bands were cut from Gel and DNA was isolated using Squeeze N Freeze (protocol see Methods)
Results:
1% Agarose Gel of new PCR-product (line 3) and cut PCR-product (line2) with 2 log ladder (line 1)
cut PCR Fragment with correct size (3,8 kbp) was frozen after isolation. PCR yielded some short products with variable length. Used PCR-Kit is not suitable for amplification of long fragments. Experiment will be repeated tomorrow with correct Kit.
 "
"