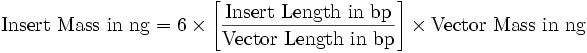Team:TU Munich/lab/notebook/part3
From 2011.igem.org
| Line 1: | Line 1: | ||
<html> | <html> | ||
<div class="ui-corner-all subcontent tabcontent"> | <div class="ui-corner-all subcontent tabcontent"> | ||
| - | < | + | <h1><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=1" title="Edit section: Light sensor systems and AND-Gate cloning Part III Alex/Simon/Bea/Thorsten">edit</a>]</span> <span class="mw-headline" id="Light_sensor_systems_and_AND-Gate_cloning_Part_III_Alex.2FSimon.2FBea.2FThorsten">Light sensor systems and AND-Gate cloning Part III Alex/Simon/Bea/Thorsten</span></h1> |
| - | + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=2" title="Edit section: 24-08-2011">edit</a>]</span> <span class="mw-headline" id="24-08-2011">24-08-2011</span></h2> | |
| - | + | <p><b>People: Alex, Simon, Bea, Thorsten</b> | |
| - | </ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | </ | + | |
| - | + | ||
| - | < | + | |
| - | </ | + | |
| - | </ | + | |
| - | < | + | |
| - | </ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
</p> | </p> | ||
| - | <h3><span class=" | + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=3" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning">Cloning</span></h3> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | </ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | </ | + | |
| - | + | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=4" title="Edit section: Ligation">edit</a>]</span> <span class="mw-headline" id="Ligation">Ligation</span></h4> | ||
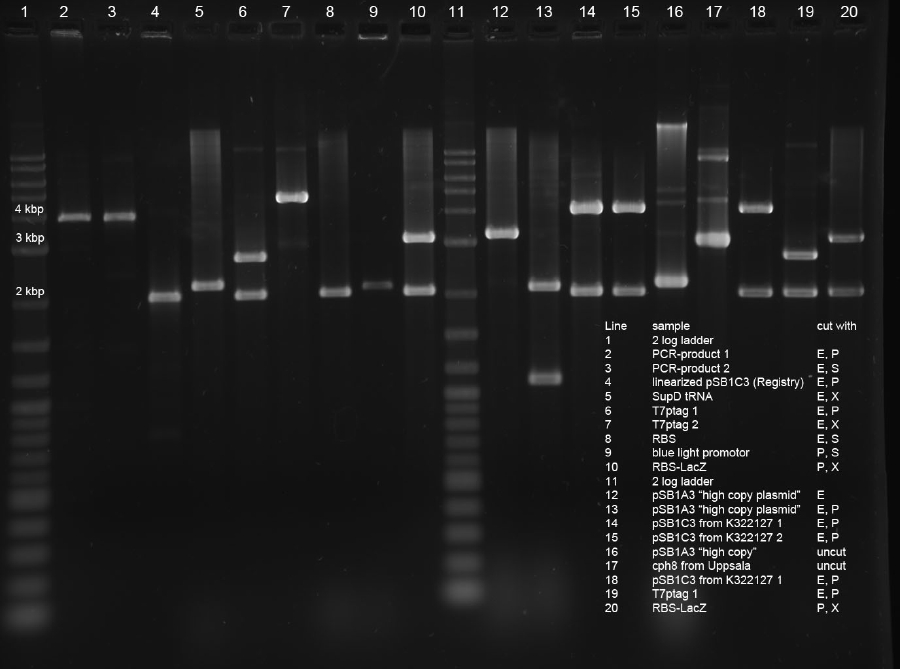
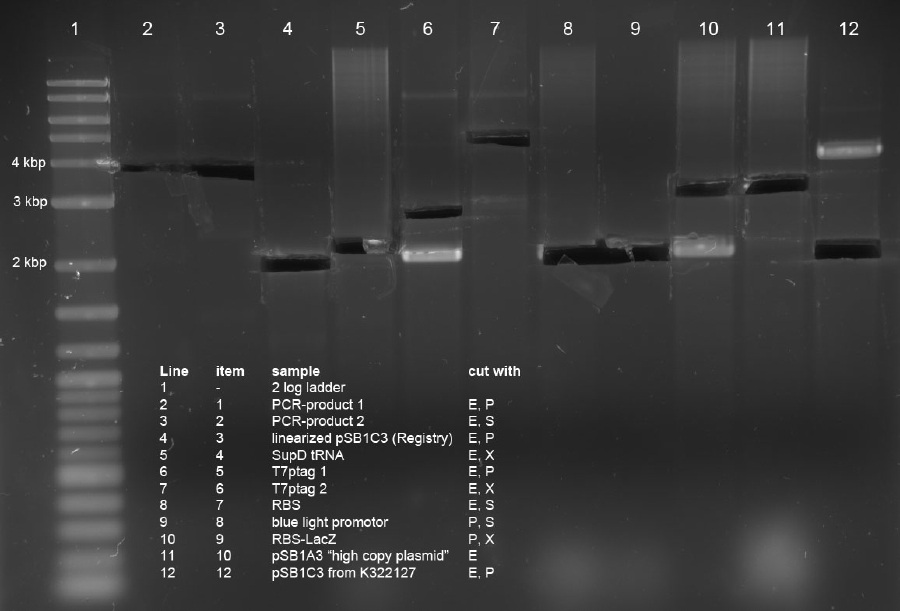
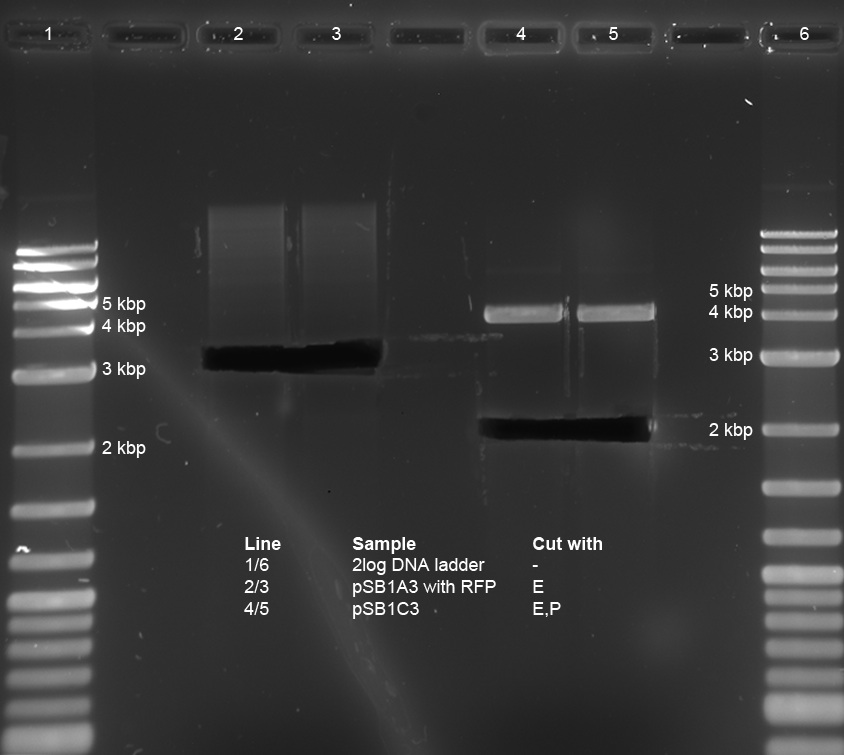
| + | <p>Three ligations in total were conducted. K238013 (S,P) was quick-ligated with lacZ (X,P), rbs (S,P) with T7Pol (X,P) and T7Prom (S,P) with lacZ (X,P). | ||
</p> | </p> | ||
| - | < | + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=5" title="Edit section: Transformation">edit</a>]</span> <span class="mw-headline" id="Transformation">Transformation</span></h4> |
| - | <p> | + | <p>The ligations were transformed into DH5alpha. Additionally, K238013 (from23-08-2011) was transformed into DH5alpha. The ligation of K238013 with lacZ is plated onto Amp and Cm, rbs with T7pol is plated onto Amp, T7Prom with lacZ onto Kan and K238013 is plated onto Amp and Cm. |
</p> | </p> | ||
| - | < | + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=6" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing">Testing</span></h3> |
| - | < | + | |
| - | |||
| - | |||
<p>The two cultures of K322127 in DH5alpha incubated over night with red light (ca. 630 nm) were split. One half was further incubated with red light, while the other half was incubated with sun light and light from a table-lamp over night. | <p>The two cultures of K322127 in DH5alpha incubated over night with red light (ca. 630 nm) were split. One half was further incubated with red light, while the other half was incubated with sun light and light from a table-lamp over night. | ||
</p><p><i>Test of negative controls on S-Gal:</i> T7prom (Kan) transformed into DH5alpha and K322127 (Cm) transformed into BL21 were inoculated onto S-Gal plates with the respective antibiotics. The are incubated over night, and should not yield black colonies, as they both should not be able to express functional lacZ. | </p><p><i>Test of negative controls on S-Gal:</i> T7prom (Kan) transformed into DH5alpha and K322127 (Cm) transformed into BL21 were inoculated onto S-Gal plates with the respective antibiotics. The are incubated over night, and should not yield black colonies, as they both should not be able to express functional lacZ. | ||
| - | |||
| - | |||
</p> | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=7" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results">Results</span></h3> | ||
| + | <p><i>Expression test of reporter plasmid:</i> The cultures with ligation of T7prom and lacZ transformed into BL21 and induced with IPTG showed black colonies on the S-Gal plates. However, the cultures, which were NOT induced also showed black colonies of the same intensity. At the current point, we cannot sufficiently explain this. | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=8" title="Edit section: Red light sensor assembly (Bea and Thorsten)">edit</a>]</span> <span class="mw-headline" id="Red_light_sensor_assembly_.28Bea_and_Thorsten.29">Red light sensor assembly (Bea and Thorsten)</span></h3> | ||
| - | |||
<p><i>aim:</i> | <p><i>aim:</i> | ||
</p><p>Ligation of red light sensor didn't work, yet. Therefore we ligated PCR product of BBa_K322127(X and P cut) into pSB3C5(S and P cut) to verify correct sites. Subsequent amplification and recutting of this part should lead to correct ligation with SupD. | </p><p>Ligation of red light sensor didn't work, yet. Therefore we ligated PCR product of BBa_K322127(X and P cut) into pSB3C5(S and P cut) to verify correct sites. Subsequent amplification and recutting of this part should lead to correct ligation with SupD. | ||
| Line 82: | Line 33: | ||
(Florian 6.7.11 labelled with "K322127 PCR product aus gel ausgeschnitten") | (Florian 6.7.11 labelled with "K322127 PCR product aus gel ausgeschnitten") | ||
</p><p><br /> | </p><p><br /> | ||
| - | |||
</p><p>pcr: | </p><p>pcr: | ||
</p><p>Template: BBa_K322127 ("Edinburgh part") conc: 150 ng/µl | </p><p>Template: BBa_K322127 ("Edinburgh part") conc: 150 ng/µl | ||
| Line 89: | Line 39: | ||
<dl><dd>BBa_K568000_fwd: tatatctatatcgaattcgcggccgcttctagagtttacggctagctcagtcctaggta (59bp) | <dl><dd>BBa_K568000_fwd: tatatctatatcgaattcgcggccgcttctagagtttacggctagctcagtcctaggta (59bp) | ||
</dd></dl> | </dd></dl> | ||
| + | |||
<dl><dd>BBa_K568000_rev: cgtgccggcggctgcagcggccgctactagtaagtccattctccccaaaaatg (53bp) | <dl><dd>BBa_K568000_rev: cgtgccggcggctgcagcggccgctactagtaagtccattctccccaaaaatg (53bp) | ||
</dd></dl> | </dd></dl> | ||
| Line 99: | Line 50: | ||
</p> | </p> | ||
<dl><dd>5 µl 10x standard buffer | <dl><dd>5 µl 10x standard buffer | ||
| - | |||
</dd></dl> | </dd></dl> | ||
<dl><dd>1 µl dNTP's | <dl><dd>1 µl dNTP's | ||
| Line 106: | Line 56: | ||
</dd></dl> | </dd></dl> | ||
<dl><dd>0,5 µl primers, respectively | <dl><dd>0,5 µl primers, respectively | ||
| + | |||
</dd></dl> | </dd></dl> | ||
<dl><dd>0,4 µl Template DNA | <dl><dd>0,4 µl Template DNA | ||
| Line 117: | Line 68: | ||
</dd></dl> | </dd></dl> | ||
<p><br /> | <p><br /> | ||
| - | |||
Bands were cut from Gel and DNA was isolated using Squeeze N Freeze (protocol see <a href="/wiki/index.php?title=Methods" title="Methods">Methods</a>) | Bands were cut from Gel and DNA was isolated using Squeeze N Freeze (protocol see <a href="/wiki/index.php?title=Methods" title="Methods">Methods</a>) | ||
</p><p><br /> | </p><p><br /> | ||
| - | < | + | </p> |
| - | </ | + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=9" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_2">Results</span></h3> |
| + | |||
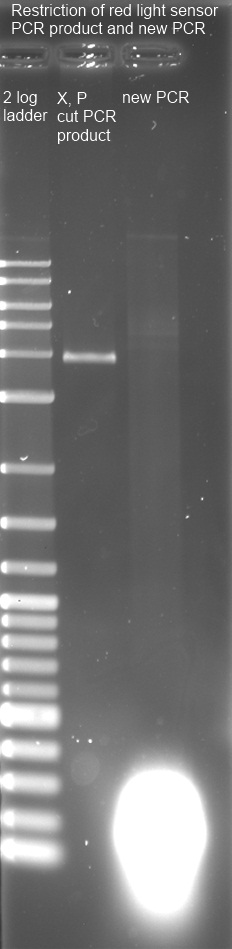
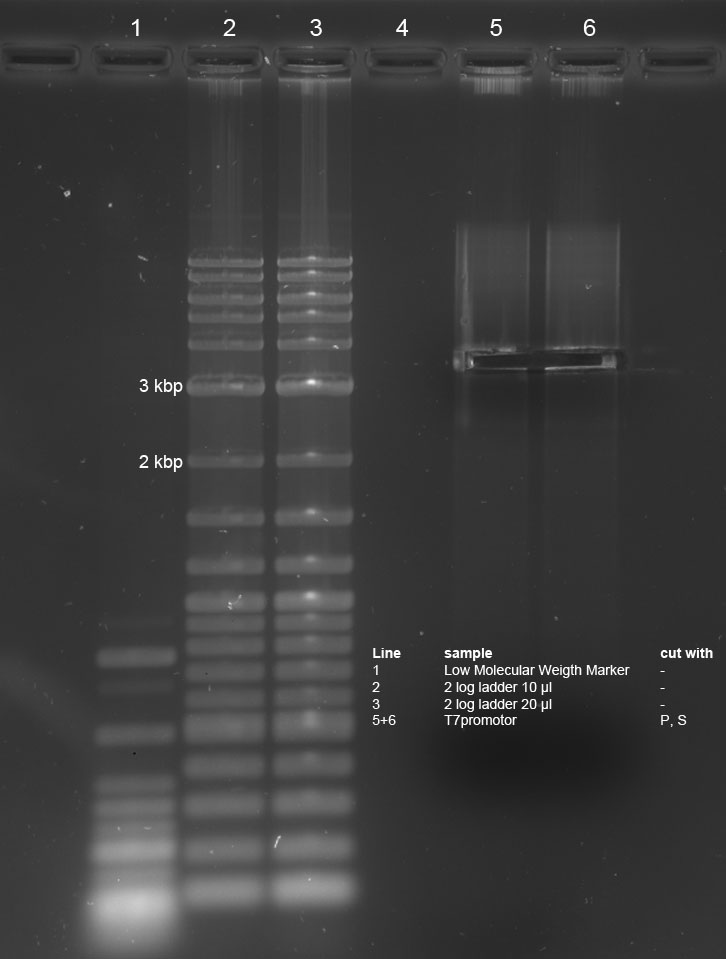
| + | <p><a href="/wiki/index.php?title=File:240811_pcr_cutXP_new_pcr_edited.jpg" class="image"><img alt="240811 pcr cutXP new pcr edited.jpg" src="/wiki/images/7/75/240811_pcr_cutXP_new_pcr_edited.jpg" width="232" height="949" /></a> | ||
</p><p>1% Agarose Gel of new PCR-product (line 3) and cut PCR-product (line2) with 2 log ladder (line 1) | </p><p>1% Agarose Gel of new PCR-product (line 3) and cut PCR-product (line2) with 2 log ladder (line 1) | ||
</p><p><br /> | </p><p><br /> | ||
</p><p>cut PCR Fragment with correct size (3,8 kbp) was frozen after isolation. | </p><p>cut PCR Fragment with correct size (3,8 kbp) was frozen after isolation. | ||
PCR yielded some short products with variable length. Used PCR-Kit is not suitable for amplification of long fragments. Experiment will be repeated tomorrow with correct Kit. | PCR yielded some short products with variable length. Used PCR-Kit is not suitable for amplification of long fragments. Experiment will be repeated tomorrow with correct Kit. | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=10" title="Edit section: 25-08-2011">edit</a>]</span> <span class="mw-headline" id="25-08-2011">25-08-2011</span></h2> | ||
| + | <p><b>People: Alex, Simon, Bea, Thorsten</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=11" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_2">Cloning</span></h3> | ||
| + | |||
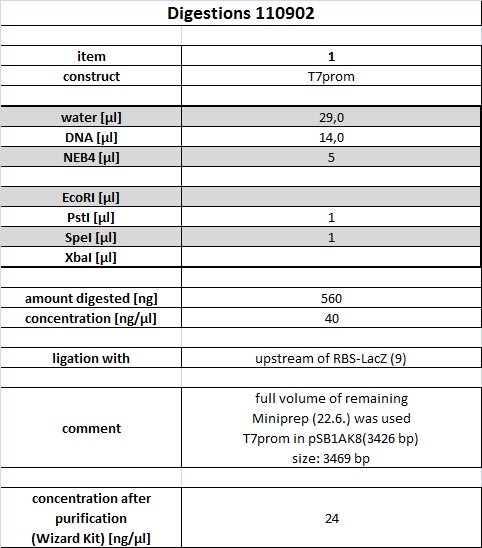
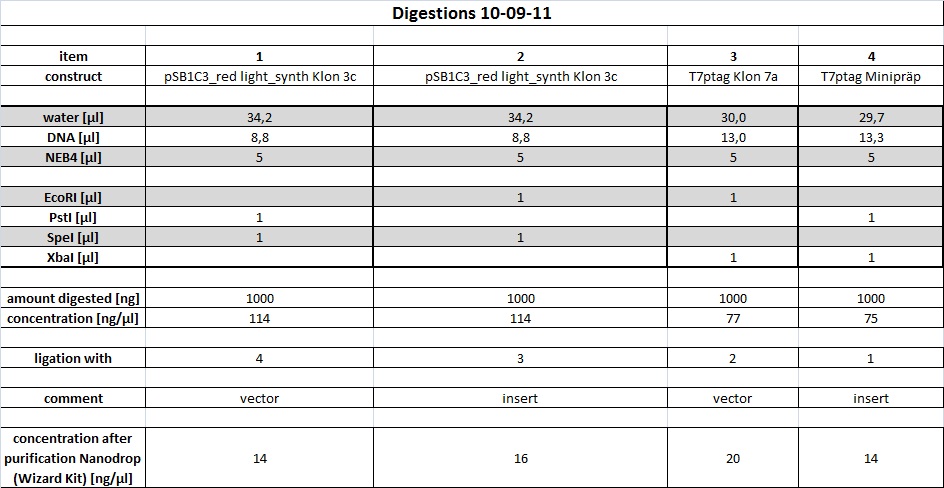
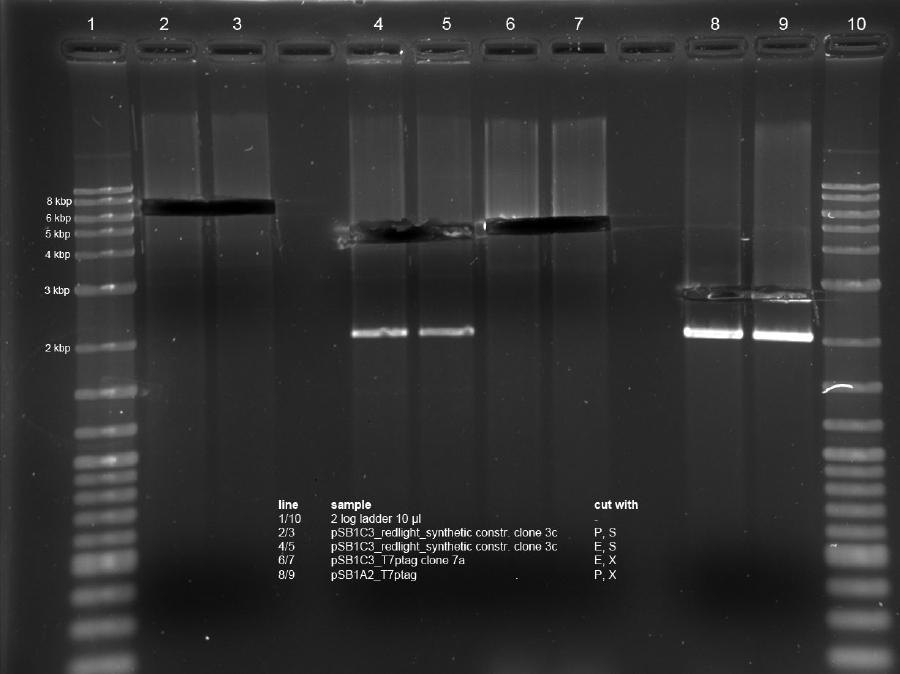
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=12" title="Edit section: Digestion">edit</a>]</span> <span class="mw-headline" id="Digestion">Digestion</span></h4> | ||
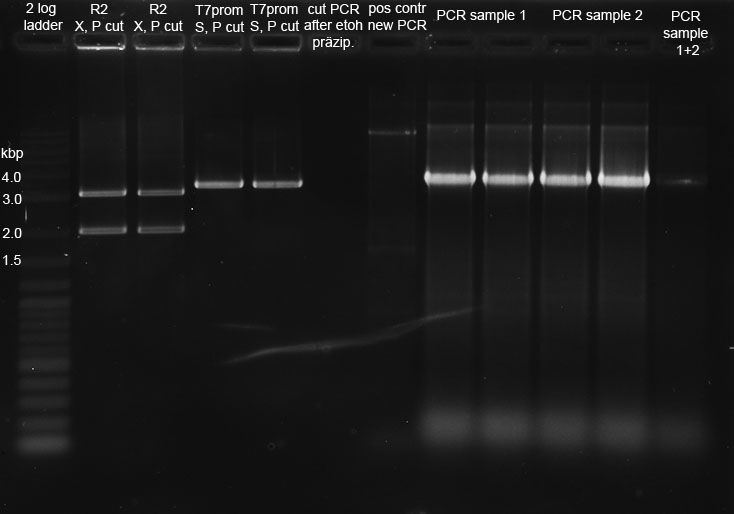
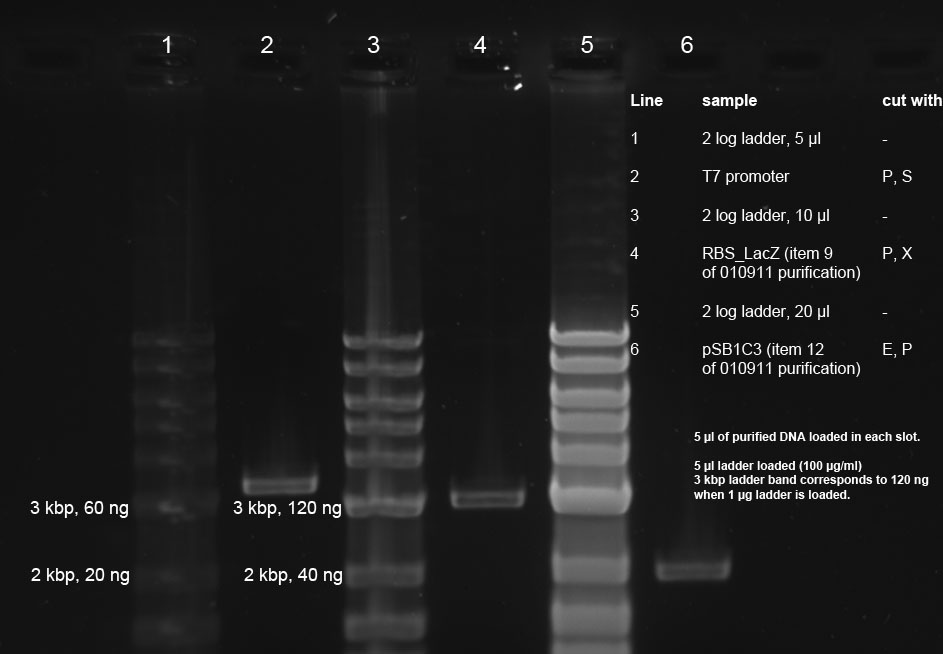
| + | <p>R2 (red writing: MiniPrep iGEM R2 31.5.11) was digested with X,P and T7prom (black writing: 17.6.11 T7) with S,P. After heat-inactivation, GLP was added to the digested DNA. The samples were applied onto a 1 % Agarose gel. The samples, along with those from the PCR (see below), were applied as follows: 2lod DNA ladder (5 ul); R2 (20 ul); R2 (20 ul); T7prom (20 ul); T7 prom (20 ul); PCR cut; positive control; PCR approach 1; PCR approach 1; PCR approach 2; PCR approach 2; PCR approaches 1 and 2 pooled. | ||
| + | The gel was run for 1:30 h at 110 V. The following bands were expected: 3.1 kbp for lacZ with rbs and 2 kbp for vector pSB1A2. 3.4 kbp for T7prom in pSB1AK8. The bands are excised from the gel and the DNA is recovered using freeze 'n squeeze. | ||
| + | </p><p><a href="/wiki/index.php?title=File:250811_restriction_and_PCR_Alex_Thorsten-1.jpg" class="image"><img alt="250811 restriction and PCR Alex Thorsten-1.jpg" src="/wiki/images/d/d6/250811_restriction_and_PCR_Alex_Thorsten-1.jpg" width="734" height="514" /></a> | ||
| + | </p><p>The original file before cutting of the bands seems to be corrupt. | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=13" title="Edit section: Ligation">edit</a>]</span> <span class="mw-headline" id="Ligation_2">Ligation</span></h4> | ||
| + | |||
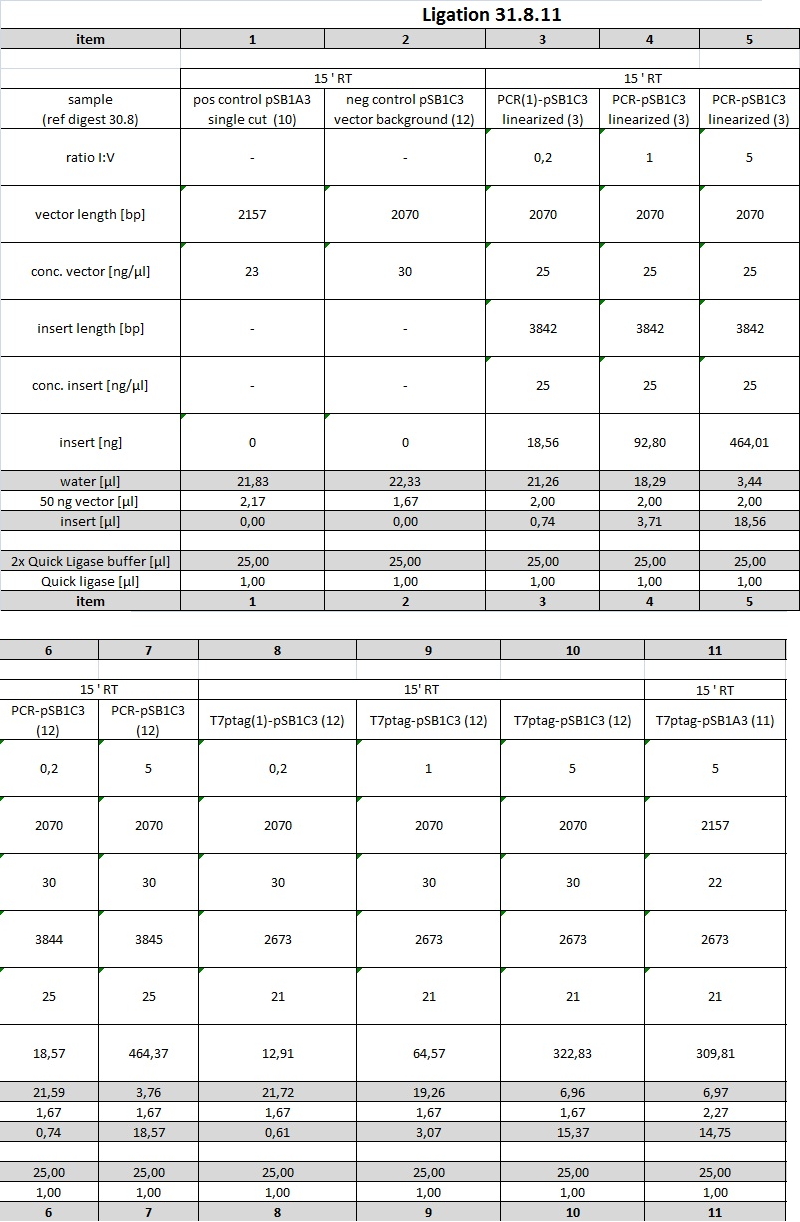
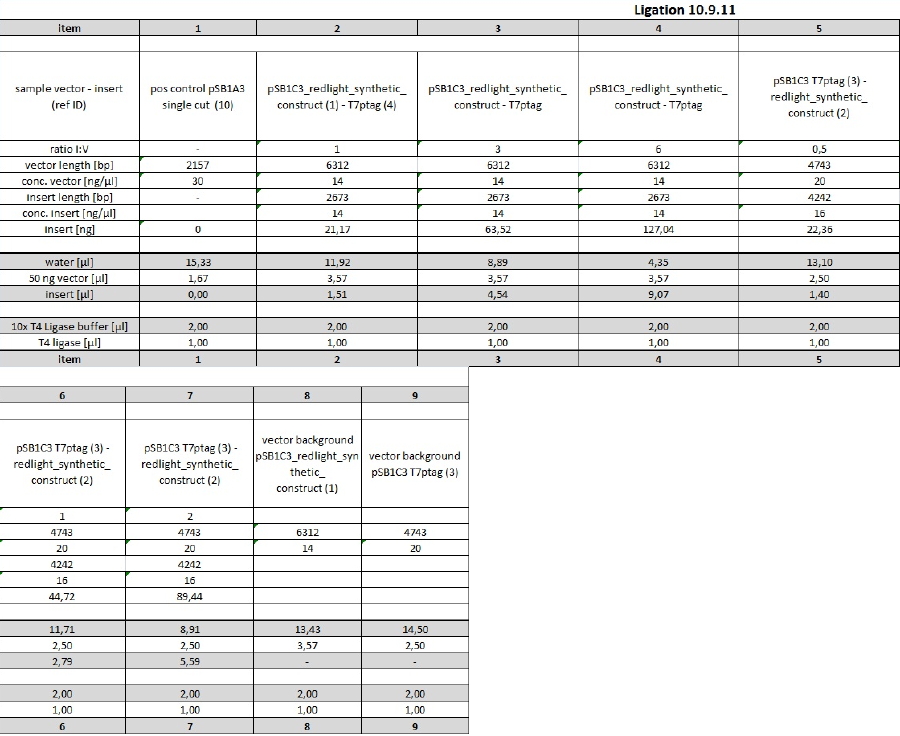
| + | <p>In the next step, K238013 (from 23-08-2011; cut with S,P) was ligated with rbs&lacZ (=R2; X,P), rbs (from 22-08-2011; S,P) with T7pol (from 23-08-2011; X,P) and T7prom (S,P) with rbs&lacZ (X,P). Each time, ligation was performed as normal and as quick-ligation. After that, the transformed cells were plated onto LB (Amp) plates and incubated over night. | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=14" title="Edit section: New PCR of K322128 using Phusion High-Fidelity PCR-Kit (NEB/Finnzymes)">edit</a>]</span> <span class="mw-headline" id="New_PCR_of_K322128_using_Phusion_High-Fidelity_PCR-Kit_.28NEB.2FFinnzymes.29">New PCR of K322128 using Phusion High-Fidelity PCR-Kit (NEB/Finnzymes)</span></h4> | ||
| + | <p><b>Material</b> | ||
| + | </p><p>BBa_K322127 with primers BBa_K568000, derived from Biomers, 3,8 kbp | ||
| + | cut vector pSB1A2 digestion with X,P from Alex on 25.8.2011 | ||
| + | (Florian 6.7.11 labelled with "K322127 PCR product aus gel ausgeschnitten") | ||
| + | </p><p><b>Procedure</b> | ||
| + | </p> | ||
| + | <pre>using same protocol as Florian on 11.7 | ||
| + | |||
| + | </pre> | ||
| + | <p><i>PCR-Program:</i> | ||
| + | </p><p>1) Initial Denaturation: 98°C, 4' | ||
| + | </p><p>2) 30 cycles of: | ||
| + | </p> | ||
| + | <pre> Denat.: 98°C, 30 s | ||
| + | Annealing: 60°C, 15 s | ||
| + | Extension: 72°C, 2´ 30 s | ||
| + | </pre> | ||
| + | <p>3) Final extension: 72°C, 8' | ||
| + | </p><p>4) Hold: 4°C, oo | ||
| + | </p><p><i>samples</i> | ||
| + | </p><p>only 2 samples were prepared using each: | ||
| + | </p><p>35,5µl of ddH2O | ||
| + | 10 µl5x buffer | ||
| + | 1 µl dNTPS | ||
| + | 1 µl of template DNA K322127 (1,5ng/µl ) | ||
| + | 1 µl of Primer fwd (10µM) | ||
| + | 1 µl of Primer rev (10µM) | ||
| + | </p><p>only the 10kB positive control was done: | ||
| + | </p><p>34µl of ddH2O | ||
| + | 10 µl5x buffer | ||
| + | 1 µl dNTPS | ||
| + | 1 µl of template DNA (template for positive control) | ||
| + | 2,5 µl of Primer (10kB-Mix) | ||
| + | |||
| + | </p><p><br /> | ||
| + | after pcr the samples were applied on a 1% Agarose-gel | ||
| + | </p><p>The bands are excised from the gel and the DNA is recovered using freeze 'n squeeze. | ||
| + | </p><p>two times 10µl of the PCR product were digested with X,P | ||
| + | </p><p>the digestion and the pcr product were stored at -20°C | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=15" title="Edit section: Ethanol precipitation of digested PCR product">edit</a>]</span> <span class="mw-headline" id="Ethanol_precipitation_of_digested_PCR_product">Ethanol precipitation of digested PCR product</span></h4> | ||
| + | <p>Material: PCR product cut with X,P from the 24.8.2011 | ||
| + | protocol see <a href="/wiki/index.php?title=Methods" title="Methods">Methods</a> | ||
| + | the sample was applied on an agarose gel because the nanodrop indicated no DNA | ||
| + | </p> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=16" title="Edit section: Result">edit</a>]</span> <span class="mw-headline" id="Result">Result</span></h4> | ||
| + | <p>The gel showed that no DNA was yielded (see picture Restriction & Ligation) | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=17" title="Edit section: Repeated Digest and Ligation of PCR Product">edit</a>]</span> <span class="mw-headline" id="Repeated_Digest_and_Ligation_of_PCR_Product">Repeated Digest and Ligation of PCR Product</span></h4> | ||
| + | <p>Material: PCR product of BBa_K322127 with primers BBa_K568000, derived from Biomers, 3,8 kbp | ||
| + | cut vector pSB1A2 digestion with X,P from Alex on 25.8.2011 | ||
| + | (Florian 6.7.11 labelled with "K322127 PCR product aus gel ausgeschnitten") | ||
| + | Procedure: | ||
| + | The PCR product was digested with X,P. After heat-inactivation, GLP was added to the digested DNA. The samples were applied onto a 0,8 % Agarose gel. The gel was run for 1:30 h at 110 V. | ||
| + | The bands are excised from the gel and the DNA is recovered using freeze 'n squeeze. | ||
| + | Ligation of the cut fragment with the vector pSB1A2 (cut with X,P; see Restriction & Ligation above) | ||
| + | using quick ligation protocol see <a href="/wiki/index.php?title=Methods" title="Methods">Methods</a> | ||
| + | |||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=18" title="Edit section: Result">edit</a>]</span> <span class="mw-headline" id="Result_2">Result</span></h4> | ||
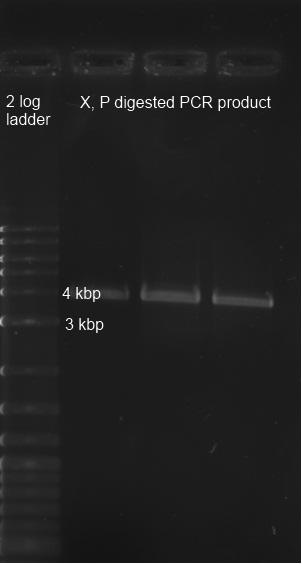
| + | <p><a href="/wiki/index.php?title=File:25082011_verdau_pcr_produkt_edited.jpg" class="image"><img alt="25082011 verdau pcr produkt edited.jpg" src="/wiki/images/c/ca/25082011_verdau_pcr_produkt_edited.jpg" width="302" height="564" /></a> | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=19" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_2">Testing</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=20" title="Edit section: SDS-PAGE">edit</a>]</span> <span class="mw-headline" id="SDS-PAGE">SDS-PAGE</span></h4> | ||
| + | |||
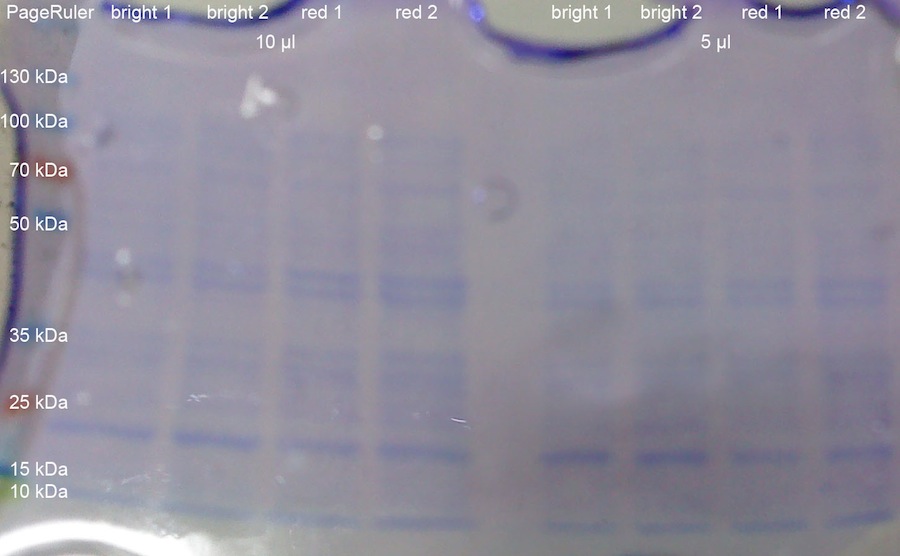
| + | <p>1 OD_600 each from the K322127 in DH5alpha cultures from yesterday was taken. The OD_600 were: 0.835 for the first colony exposed to bright lamp (bright 1); 0.84 for the second colony exposed to bright lamp (bright 2); 1.685 for the first colony incubated with red light (red 1); 1.855 for the second colony incubated with red light (red 2). The cells were centrifuged at 13000 rcf for 8 min. After resuspension in 100 ul loading buffer, the tubes were incubated for 10 min at 95 °C. After that, the samples (5 ul and 10 ul) were loaded onto a 10 % SDS-PAGE with stacking gel. Pageruler was used as protein ladder. The gel was run for 2:00 h at 80 V, which was increased to 120 V when the buffer front reaching the separation gel. After that, the gel was stained with Coomassie blue and subsequently destained. | ||
| + | </p><p><a href="/wiki/index.php?title=File:110825_SDS-PAGE_von_Alex.jpg" class="image"><img alt="110825 SDS-PAGE von Alex.jpg" src="/wiki/images/3/32/110825_SDS-PAGE_von_Alex.jpg" width="1454" height="898" /></a> | ||
| + | </p><p>The gel does not show elevated intensities of the band at around 10 kDa for lacZ alpha peptide, which would have been expected. | ||
| + | </p> | ||
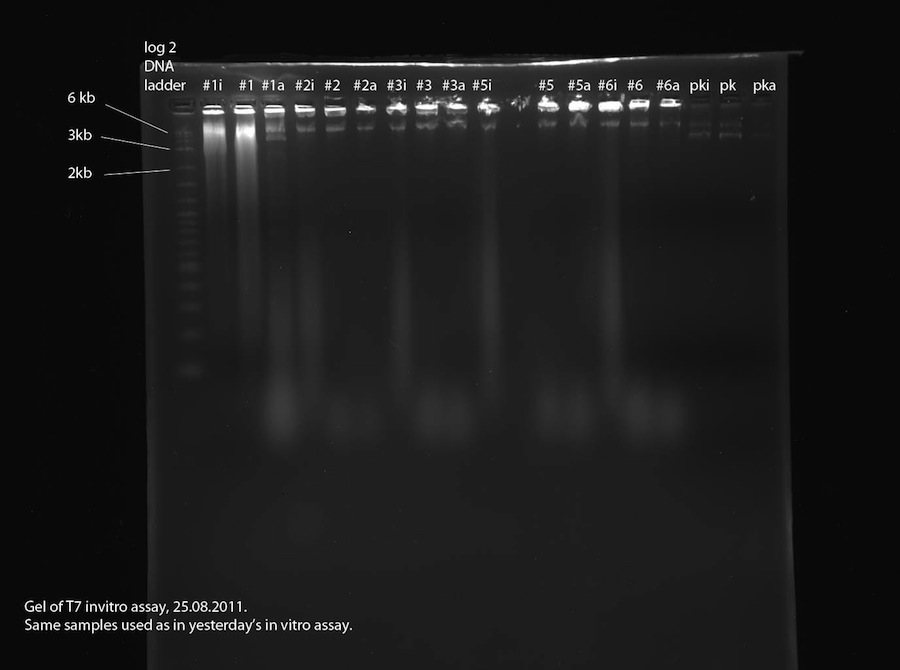
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=21" title="Edit section: T7 promoter in vitro assay">edit</a>]</span> <span class="mw-headline" id="T7_promoter_in_vitro_assay">T7 promoter in vitro assay</span></h4> | ||
| + | <p>As proposed yesterday, a second in vitro assay was performed to find out if the plasmids contain a T7 promoter. This time, a third sample was made for each plasmid (same plasmids as yesterday, see above). In this sample (labeled "#xi") the RNase that we assume to be present were inhibited using RNA secure prior to addition of T7 polymerase and incubation. See <a href="/wiki/index.php?title=Methods" title="Methods">methods</a> for detailed procedure. The 2% agarose gel was run at 110 V, 400 mA for 1:30 hours. | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=22" title="Edit section: Other Work">edit</a>]</span> <span class="mw-headline" id="Other_Work">Other Work</span></h3> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=23" title="Edit section: K322127 Inoculation & Incubation:">edit</a>]</span> <span class="mw-headline" id="K322127_Inoculation_.26_Incubation:">K322127 Inoculation & Incubation:</span></h4> | ||
| + | <p>The following inoculations were performed: | ||
| + | </p><p>- "K238013 in DH5 alpha", plate date: 24.08.2011, yesterday's transformation, in LB Amp | ||
| + | </p><p>- "BM28 from Jeanette Winter", plate date: 31.05.2011, in LB Amp, Kan | ||
| + | </p><p>- "green light 2", plate date: 27.6.2011, in LB Cm | ||
| + | </p><p>- "green light 3", plate date: 27.6.2011, in LB Cm | ||
| + | </p><p>- "green light 4", plate date: 27.6.2011, in LB Cm | ||
| + | </p><p>- "K322127 in DH5 alpha, Kolonie 1, Licht-induziert", plate date: 22.08.11, in LB cm (the picked colonies were the black ones after over night incubation in light) | ||
| + | </p><p>- "K322127 in DH5 alpha, Kolonie 2, Rotlicht-deaktiviert", plate date: 22.08.11, in LB cm (the picked colonies were white ones after over night incubation in red light) | ||
| + | </p><p>Incubation over night at 37 °C, 200 rpm. | ||
| + | |||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=24" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_3">Results</span></h3> | ||
| + | <p>The PCR was successful (see picture Restriction & Ligation), the positive control and both samples yielded DNA that is located at the right spot according to their length | ||
| + | positive control (10kB) | ||
| + | samples (3,8kB) | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=25" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_4">Results</span></h3> | ||
| + | |||
| + | <p><i>24.08.11</i> | ||
| + | </p><p><i>T7 promoter in vitro assay</i> | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:250811_invitroassay3bearbeitet.jpg" class="image"><img alt="250811 invitroassay3bearbeitet.jpg" src="/wiki/images/3/31/250811_invitroassay3bearbeitet.jpg" width="1376" height="1024" /></a> | ||
| + | </p><p><br /> | ||
| + | </p><p>The samples labeled "#xi" were treated with RNA secure to inhibit potentially present RNase. The gel photo shows blurry signals in all of these lanes which aren't visible in lanes "#x" and "#xa". This proves that RNase is present in the DNA samples used in the assay. Also, the fact that the RNA signals are quite large and strong shows leads us to the conclusion that the examined plasmids all contain a T7 promoter. Sample 1 contains two plasmids (about 6.6 kb and 3.5 kb) which is the reason for the bad sequencing data. Samples 2, 3, 5 and 6 contain one 6.6 kb plasmid each. This should be our correct reporter plasmid, part 2b, containing T7 promoter and lacZ. To verify this, a sample shall be sequenced. | ||
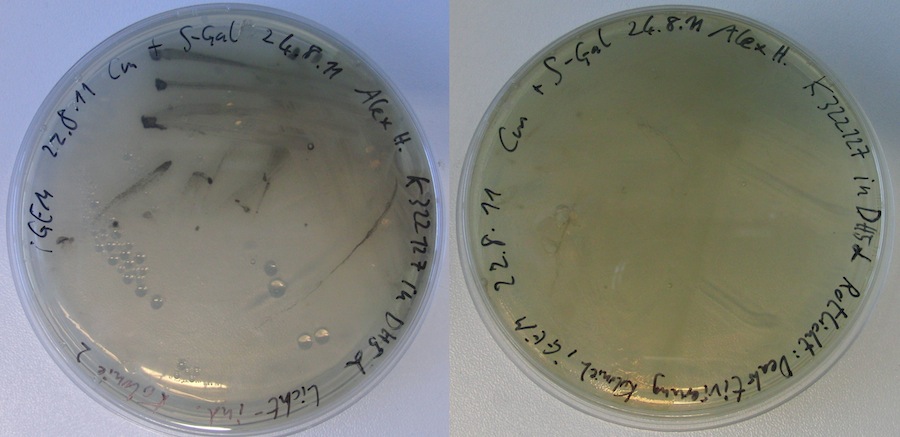
| + | </p><p><i>S-Gal negative Control:</i> | ||
| + | </p><p>DH5alpha cells transformed with T7prom yielded white colonies on the S-Gal plates. However, BL21 cells transformed with K322127 yielded all black colonies. Most likely, our strain of BL21 is unsuited for expression tests using S-Gal (might constitutively express lacZ). | ||
| + | </p><p><i>Expression test of K322127</i> | ||
| + | </p><p>After over night incubation, both plates incubated in red light showed white colonies only. Of the two plates incubated under the lamp, one did not show any colonies. The other one showed black colonies. | ||
| + | --> First evidence that part K322127 works as expected!!! To further quantify and characterise this: Miller assay? Other methods? | ||
| + | </p><p><a href="/wiki/index.php?title=File:240811_K322127inDH5alpha_Kolonie2.jpg" class="image"><img alt="240811 K322127inDH5alpha Kolonie2.jpg" src="/wiki/images/8/89/240811_K322127inDH5alpha_Kolonie2.jpg" width="5136" height="2496" /></a> | ||
| + | |||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=26" title="Edit section: 26-08-2011">edit</a>]</span> <span class="mw-headline" id="26-08-2011">26-08-2011</span></h2> | ||
| + | <p><b>People: Alex, Simon, Bea, Thorsten</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=27" title="Edit section: Other Work">edit</a>]</span> <span class="mw-headline" id="Other_Work_2">Other Work</span></h3> | ||
| + | <p><b>Part-request:</b> Parts requested from the registry for backup if the ligation of the PCR product will fail again: | ||
| + | |||
| + | </p> | ||
| + | <table class="wikitable"> | ||
| + | |||
| + | <tr> | ||
| + | <th>Part </th><th>Team </th><th>Library </th><th>Plate </th><th>Well </th><th>Plasmid </th><th>Resistance | ||
| + | </th></tr> | ||
| + | <tr> | ||
| + | <td>BBa_K322123 </td><td>iGEM10_Edindburgh </td><td>2010 Submisions Samples 001 </td><td>Shipment: 00694 </td><td>4D </td><td>pSB1C3 </td><td>C | ||
| + | |||
| + | </td></tr> | ||
| + | <tr> | ||
| + | <td>BBa_K322124 </td><td>iGEM10_Edindburgh </td><td>2010 Submisions Samples 001 </td><td>Shipment: 00694 </td><td>4E </td><td>pSB1C3 </td><td>C | ||
| + | </td></tr></table> | ||
| + | <p>And Gate plasmid: | ||
| + | PCR-Product from Edinburgh | ||
| + | </p><p><b>New media and plates:</b> | ||
| + | LB, LB for Agar plates (Kan and Amp?) and Luria medium were prepared. Plates were prepared. | ||
| + | </p><p><b>Sequencing:</b> | ||
| + | </p><p>The reporter plasmid clone 5 was handed in for sequencing at GATC-Biotech. The primer used for sequencing is "Primer pSB1A2 und pSB1AK8 fwd". | ||
| + | |||
| + | </p><p><b>Skype talk with iGEM Team Freiburg</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=28" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_3">Cloning</span></h3> | ||
| + | <p><b>Miniprep & Glycero-Stock:</b> The cultures inoculated yesterday on LB were frozen at -80 °C as glycero-stocks. The culture of K238013 (pSB1A2) in DH5alpha was prepped. The resulting DNA yielded a concentration of approximately 300 ng/ul. | ||
| + | </p><p><b>Repetition of transformations:</b> | ||
| + | </p><p>The transformations from yesterday (ligation using ligation protocol) were repeated using DH5alpha cells (kindly provided by Andrea). Transformations were done for reporter plasmid (part 2b) colony 5 from DATUM, K238013 with lacZ, rbs with T7pol and T7prom with lacZ. After incubation for one hour, the cells were plated on LB Amp (K238013 with lacZ; rbs with T7pol) and on LB Kan (reporter plasmid; T7prom with lacZ) and incubated over night. | ||
| + | |||
| + | </p><p><b>Ligation of new PCR product with pSB1A2</b> | ||
| + | </p><p><i>Material:</i> | ||
| + | </p><p>cut PCR (X,P) product from K322127 from PCR 25.8.2011 | ||
| + | cut pSB1A2 (X,P) from Alex from 25.8. | ||
| + | </p><p><i>procedure: </i> | ||
| + | </p><p>we purified PCR product using Zymo DNA Clean +Concentrator 25 | ||
| + | </p><p><i>ligation:</i> | ||
| + | </p><p>sample 1: 15µl PCR product 5µl vector | ||
| + | sample 2: 10µl PCR product 10µl vector | ||
| + | </p><p><i>Transformation of the ligation and R2 as transformation control:</i> | ||
| + | </p><p>the two samples from purifiying and the part R2( OmpR in pSB1A2) as a positive control were transformed via electroperforation in competent DH5alpha cells that were kindly supplied by Andrea Mückl. | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=29" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_3">Testing</span></h3> | ||
| + | |||
| + | <p><b>Testing K322127</b> | ||
| + | </p><p>Material: inoculated cultures of DH5alpha with K322127 from 25.8 | ||
| + | Procedure: 100 µl of the culture was spread on S-gal plates with Kan and amp in dark | ||
| + | Incubation of the plates at room temperature (30°C) under light of diode with maximum of 630nm (turn-off diode) for 2 hours | ||
| + | afterwards incubation at light at 37°C for 19 hours afterwards 4°C in the incubator: | ||
| + | one culture with aluminium TUM logo put on top | ||
| + | one culture in the box under light of turn-off diode (negative control) | ||
| + | one culture on top of the box, light from turn-off diode from below, light from the incubator from top only on cut out TUM logo in aluminium foil | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=30" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_5">Results</span></h3> | ||
| + | <p><i>26-08-2011</i> | ||
| + | </p><p><b>Transformations:</b> The transformations from yesterday, including K238013 & lacZ, rbs & T7pol, T7prom & lacZ as well as PCR product & Vector, did not work at all. There were no colonies on the plates. | ||
| + | |||
| + | </p><p><b>Sequencing:</b> More data from the sequencing from 19-08-2011 arrived. The newly ligated K238013 & double terminator from 17-08-2011 does contain the double terminator. However, as witnessed before, the blue light promotor K238013, which was part of the plasmid prior to ligation, is missing. We are still awaiting the data from another sequencing from the same construct, but from another ligation. | ||
| + | </p><p><b>Planning for next week:</b> We need to prepare new electrocompetent DH5alpha cells. EVERYONE, who has free time to spare, please come to the lab and help. We might also be able to get new BL21 (DE3) cells from Prof. Buchner. If anyone wants to get them, contact Lars Mitschke at the Lehrstuhl of Prof. Buchner and talk to him about the request, which Alex asked about. BL21 (DE3) contain a T7polymerase, which can be induced by IPTG. This would be great use in order to test our reporter plasmid, i case the ligation worked (which we should know on Monday). | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=31" title="Edit section: 29-08-2011">edit</a>]</span> <span class="mw-headline" id="29-08-2011">29-08-2011</span></h2> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=32" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_4">Cloning</span></h3> | ||
| + | |||
| + | <p><b>inoculation</b> | ||
| + | the following were inoculated: | ||
| + | T7 Promotor with lacZ | ||
| + | 2x K322127 in DH5alpha | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=33" title="Edit section: Retransformation of reporter plasmid clone 5">edit</a>]</span> <span class="mw-headline" id="Retransformation_of_reporter_plasmid_clone_5"> Retransformation of reporter plasmid clone 5</span></h4> | ||
| + | <p>the reporter plasmid clone 5 (118ng/µl) was diluted to 50 pg/µl and to 20 ng/µl | ||
| + | </p><p>1 µl of each dilution was transformed into DH5alpha | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=34" title="Edit section: Transformation of RBS">edit</a>]</span> <span class="mw-headline" id="Transformation_of_RBS">Transformation of RBS</span></h4> | ||
| + | |||
| + | <p>0,42 µl of RBS (47,5 ng/µl) were transformed into DH5alpha | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=35" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_6">Results</span></h3> | ||
| + | <p><i>26-08-2011</i> | ||
| + | </p><p><b>transformations</b> | ||
| + | </p><p>were only successful for R2, T7 Promotor with lacZ, reporter plasmid colony 5 | ||
| + | </p><p><b>testing of K322127</b> | ||
| + | </p><p>only very small colonies on the plate with no coloring | ||
| + | plate that was illuminated with turn-off light and light from the incubator dried out. no black colonies could be detected. Incubation of plate with TUM pattern was continued. Plate was irradated with a light bulb. | ||
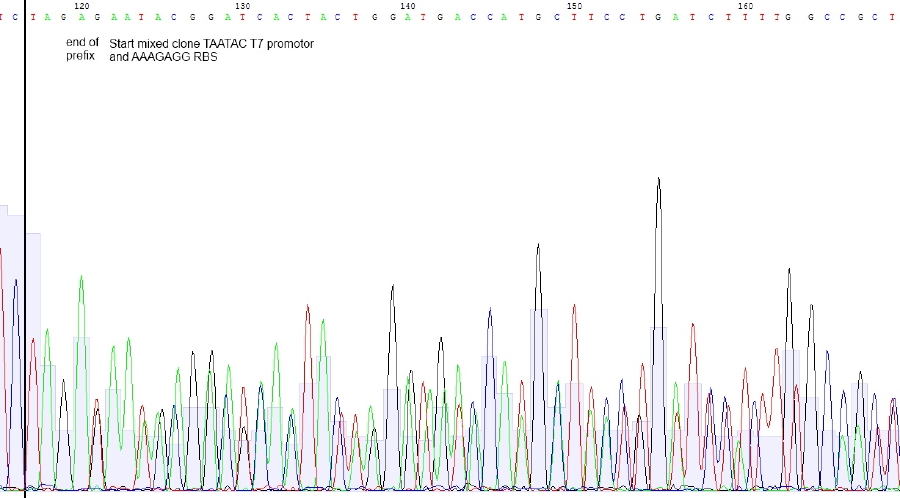
| + | </p><p><b>sequencing of reporter plasmid colony 5</b> | ||
| + | |||
| + | </p><p><a href="/wiki/index.php?title=File:Sequencemixedclone.jpg" class="image"><img alt="Sequencemixedclone.jpg" src="/wiki/images/2/29/Sequencemixedclone.jpg" width="395" height="310" /></a> | ||
| + | </p><p>Bases in squared brackets are optional and belonging to another sequence. Due tu overlapping base shifts exact assignment of bases is difficult | ||
| + | </p><p><a href="/wiki/index.php?title=File:Sequencemixedclonechromatogram.jpg" class="image" title="Sequencemixedclonechromatogram.jpg"><img alt="Sequencemixedclonechromatogram.jpg" src="/wiki/images/8/80/Sequencemixedclonechromatogram.jpg" width="1226" height="679" /></a> | ||
| + | </p><p>Sequencing reveals mixed clones. Correct Sequence with T7 promotor - RBS – lacZ is probably present beneath construct with T7 promotor lacking. Preparation of RBS-LacZ vector was contaminated with uncut or religated vector. Since plasmid length was ok, construct should be present. | ||
| + | </p><p>next steps: | ||
| + | </p><p>Retransformation with 50 pg and 20 ng MiniPrep DNA will be performed with subsequent sequencing of 4 clones. | ||
| + | In parallel new construct from Alex will be sequenced. | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=36" title="Edit section: 30-08-2011">edit</a>]</span> <span class="mw-headline" id="30-08-2011">30-08-2011</span></h2> | ||
| + | <p><b>People: Thorsten, Bea</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=37" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_7">Results</span></h3> | ||
| + | |||
| + | <p><i>29.8.11</i> | ||
| + | </p><p>Re-Transformation of part 2b clone 5 with 20 ng vector was succesful. 50 pg vector didnt yield any colonies. RBS was tranformed succesfully. 5 ml LB o/n cultures with apropriate antibiotica were inoculated and incubated at 37° C shaking. Mini Prep and sequencing will be performed at 31.8.11 | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=38" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_5">Cloning</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=39" title="Edit section: Electrocompetent cells">edit</a>]</span> <span class="mw-headline" id="Electrocompetent_cells">Electrocompetent cells</span></h4> | ||
| + | <dl><dd>new electrocompetent cells (DH5alpha) were made by the following protocol: | ||
| + | |||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>a 20 ml Luria medium overnight culture was inoculated with electrocompetent DH5alpha (kindly provided Andrea Mückl) yesterday | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>350 ml Luria medium were inoculated with 15 ml of the overnight culture and incubated at 37°C until a OD(600) of 0,7 was reached | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>the medium was distributed on falcons and the cells were centrifuged at 4500 rpm for 20 min at 4°C | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>after discarding the supernatant 350 ml of sterile ice-cold 10% glycerol was added and the pellets were resuspended using a pipet | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>the resuspension was centrifuged at 4500 rpm at 4°C for 11 minutes | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>after discarding the supernatant the cells were resuspended again in 350 ml of ice-cold 10% glycerol ( cells kept chilled all the time) | ||
| + | |||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>and again centrifuged at 4500 rpm at 4°C for 11 minutes | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>the supernatant was discarded and the cells were resuspended in the remaining supernatant | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>the cells were dispensed in 40 µl aliquots and stored at -80°C for further use | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=40" title="Edit section: Planning">edit</a>]</span> <span class="mw-headline" id="Planning">Planning</span></h4> | ||
| + | |||
| + | <p>In order to get ligations working, purification steps will be performed using Promegas Wizard PCR and Gel clean up Kit. | ||
| + | We are going to ligate PCR product into pSB1C3 and upstream of SupD-tRNA. In parallel, T7ptag will be ligated into AND-Gate Plasmid, which will be pSB1C3. [First step of assembly] | ||
| + | </p><p>Then, synthesis product, which contains parts from SupDtRNA to RBS J44001, will be ligated into succesful AND-Gate Plasmid-T7ptag. The last step will be ligation of PCR-product into this construct. | ||
| + | </p><p>Excel sheets can be found in DropBox folder "protocols". | ||
| + | </p><p>planned parts | ||
| + | </p><p>PCR product ligated into pSB1C3 will be a new part "red light sensor without lacZ". | ||
| + | </p><p>PCR product ligated with SupD will be a new part as well. | ||
| + | </p><p>Synthesis product is another maybe not so useful part which will be send into the registry. | ||
| + | </p><p>Reporter plasmid will be another Part. This one was called "part 2B" in recent cloning steps and needs further validation (see 29.8. retransformation and sequencing) | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=41" title="Edit section: Restriction digest">edit</a>]</span> <span class="mw-headline" id="Restriction_digest">Restriction digest</span></h4> | ||
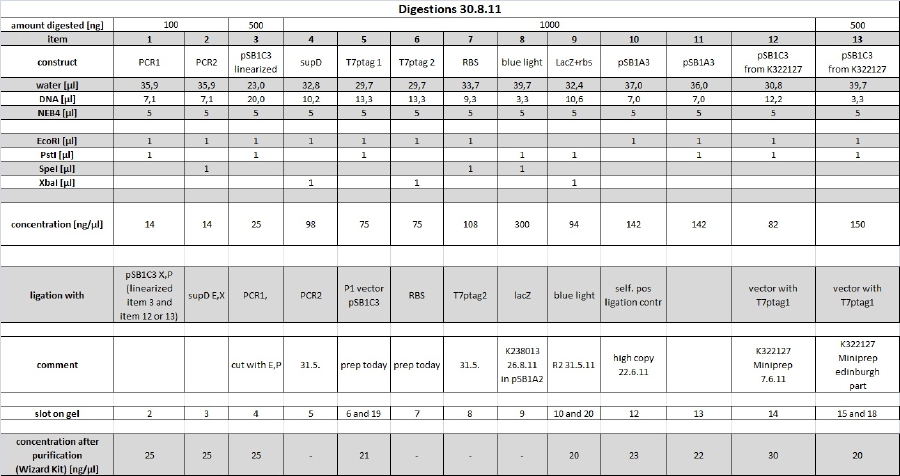
| + | <p><a href="/wiki/index.php?title=File:Digest_30811_tho.jpg" class="image"><img alt="Digest 30811 tho.jpg" src="/wiki/images/d/da/Digest_30811_tho.jpg" width="1292" height="684" /></a> | ||
| + | </p><p><br /> | ||
| + | |||
| + | <a href="/wiki/index.php?title=File:300811_digestions_tho.jpg" class="image"><img alt="300811 digestions tho.jpg" src="/wiki/images/b/bc/300811_digestions_tho.jpg" width="1376" height="1024" /></a> | ||
| + | </p><p><br /> | ||
| + | All digestions were complete and running on the expected lengths. | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=42" title="Edit section: Purification of restricion digests from gel with Wizard Kit">edit</a>]</span> <span class="mw-headline" id="Purification_of_restricion_digests_from_gel_with_Wizard_Kit">Purification of restricion digests from gel with Wizard Kit</span></h4> | ||
| + | <p>the following items were purified using the Wizard Kit according to manufacturer's instructions and the concentration was determined by nanodrop: | ||
| + | </p><p>1: 24,5 ng/µl | ||
| + | </p><p>2: 24,5ng/µl | ||
| + | </p><p>3: 24,5 ng/µl | ||
| + | </p><p>5: 20,5 ng/µl | ||
| + | </p><p>9: 20,0 ng/µl | ||
| + | </p><p>10: 23 ng/µl | ||
| + | |||
| + | </p><p>11: 21,5ng/µl | ||
| + | </p><p>12: 29,5 ng/µl | ||
| + | </p><p>13: 20,0 ng/µl | ||
| + | </p><p>for more details see restriction digest table | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=43" title="Edit section: Inoculation">edit</a>]</span> <span class="mw-headline" id="Inoculation">Inoculation</span></h4> | ||
| + | <p>the following overnight cultures were inoculated: | ||
| + | </p><p>RBS | ||
| + | reporter plasmid clone 1-5 | ||
| + | K322127 from plate from 24.8.2011 | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=44" title="Edit section: 31-08-2011">edit</a>]</span> <span class="mw-headline" id="31-08-2011">31-08-2011</span></h2> | ||
| + | |||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=45" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_8">Results</span></h3> | ||
| + | <p><i>30.8.11</i> | ||
| + | </p><p>Electrocompetent cells show good transformation efficiency. 100µl 10µl and 1µl of test transformation of 30 ng BBa_K322127 were plated on LB/cam plates. 1 µl yielded ca 400 colonies which refers to a tranformationefficiency of 1.3*10^7 cfu/µg BBa_K322127 in pSB1C3. 1*10^8 cfu/µg pUC19 is expected, so 1.3*10^7 cfu/µg vector with insert seems to be ok. | ||
| + | </p><p>cph8 from Uppsala was succesfully transformed into DH5alpha. Cells have been 1:3 diluted and 20 ng plasmid was used. Output was ca 10^6 - 10^7 cfu/µg. Plate will be stored at 4°C til inoculation of o/n culture with subsequent minipreparation. | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=46" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_6">Cloning</span></h3> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=47" title="Edit section: Ligation">edit</a>]</span> <span class="mw-headline" id="Ligation_3">Ligation</span></h4> | ||
| + | <p><a href="/wiki/index.php?title=File:31811_ligation_tho.jpg" class="image"><img alt="31811 ligation tho.jpg" src="/wiki/images/3/36/31811_ligation_tho.jpg" width="1688" height="611" /></a> | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=48" title="Edit section: Minipreparation of DNA">edit</a>]</span> <span class="mw-headline" id="Minipreparation_of_DNA">Minipreparation of DNA</span></h4> | ||
| + | <p>The following concentrations were gained through minipreparation: | ||
| + | </p><p><br /> | ||
| + | RBS : 86,5 ng/µl | ||
| + | |||
| + | </p><p>reporter plasmid clone 1: 135 ng/µl | ||
| + | </p><p>reporter plasmid clone 2: 234 ng/µl | ||
| + | </p><p>reporter plasmid clone 3: 102 ng/µl | ||
| + | </p><p>reporter plasmid clone 4: 119 ng/µl | ||
| + | </p><p>reporter plasmid clone 5: 96 ng/µl | ||
| + | </p><p><br /> | ||
| + | The reporter plasmid clone 1-5 were sent to be sequenced at GATC. | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=49" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_4">Testing</span></h3> | ||
| + | <p>the overnight culture of K322127 was used to inoculate a 100 ml culture in LB which was incubated for 5 hours at 37°C | ||
| + | </p><p>at an OD of 1.6 50 ml of the cells were centrifuged at 4500 rpm the supernatant discarded | ||
| + | </p><p>the cells were resuspended in remaining 1 ml of LB | ||
| + | |||
| + | </p><p>100 ml of LB media with s-gal was prepared and autoclaved according to protocol see Methods | ||
| + | </p><p>the resuspended cells were added to the medium when it reached 40°C | ||
| + | </p><p>the media was given in thee plates | ||
| + | </p><p>two plates were incubated at 37°C under turn-off light (diode max. 630 nm) | ||
| + | </p><p>the TUM cookie cutters were inserted in one plate that was then incubated under the light of a lamp | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=50" title="Edit section: Other Work">edit</a>]</span> <span class="mw-headline" id="Other_Work_3">Other Work</span></h3> | ||
| + | <p>new chloramphenicol plates were made | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=51" title="Edit section: 01-09-2011">edit</a>]</span> <span class="mw-headline" id="01-09-2011">01-09-2011</span></h2> | ||
| + | |||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=52" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_7">Cloning</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=53" title="Edit section: gel purification of 30.8.11 digest">edit</a>]</span> <span class="mw-headline" id="gel_purification_of_30.8.11_digest">gel purification of 30.8.11 digest</span></h4> | ||
| + | <p>20 µl of remaining digestions (made on 30.8.11) were loaded on 1 % agarose gel with 1xTAE. Items 1,2,3,4,5,6,7,8,9,10,12 were used. | ||
| + | This samples were cut using 350nm UV illumination as short as possible. Cut gel slices were subsequently purified | ||
| + | using Promegas Wizard Kit according to manufacturers instructions. | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:010911_digestions_thorsten_cut.jpg" class="image"><img alt="010911 digestions thorsten cut.jpg" src="/wiki/images/5/5f/010911_digestions_thorsten_cut.jpg" width="1156" height="785" /></a> | ||
| + | </p><p><br /> | ||
| + | |||
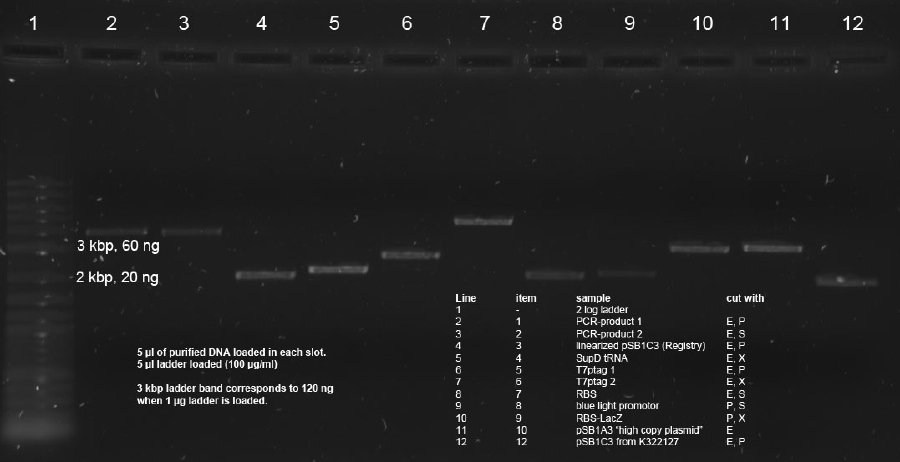
| + | Determination of concentration with Nanodrop resultet in not reliable data. Therefore quantitative 0.7 % agarose gel was run with 5 µl of each sample. 5 µl 2 log ladder was used (100 µg/ml). PCR Band (3.8 kbp) in line 2 was estimated to 75 ng. This gives aproximately 15 ng/µl. Intensities of 2 kbp band in line 4 as well as lines 5, 6, 7, 10 and 11 correspond to approx. 150 ng amount (--> 30 ng/µl). | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:010911_quantitative_gel.jpg" class="image"><img alt="010911 quantitative gel.jpg" src="/wiki/images/f/f6/010911_quantitative_gel.jpg" width="1077" height="553" /></a> | ||
| + | </p><p><br /> | ||
| + | Due to fly-by-night intensity of ladder those concentrations will be determined again on 02.09.11 for further ligations. Ligations today will be done with the following concentrations | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:110901_concentrations_analytical_gel_tho.jpg" class="image"><img alt="110901 concentrations analytical gel tho.jpg" src="/wiki/images/b/b6/110901_concentrations_analytical_gel_tho.jpg" width="1170" height="140" /></a> | ||
| + | </p><p><br /> | ||
| + | </p> | ||
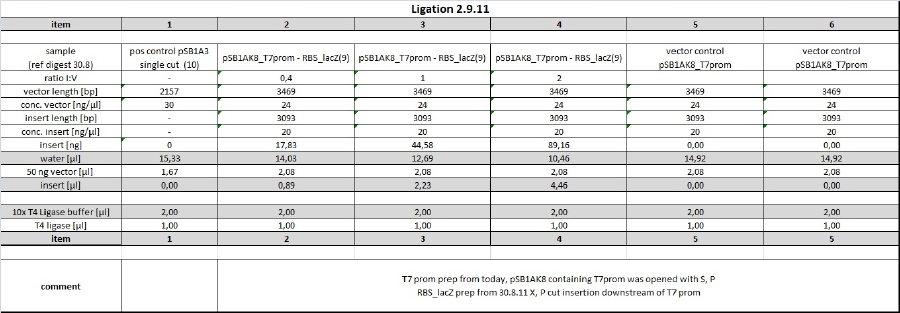
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=54" title="Edit section: o/n ligation with T4 Ligase">edit</a>]</span> <span class="mw-headline" id="o.2Fn_ligation_with_T4_Ligase">o/n ligation with T4 Ligase</span></h4> | ||
| + | |||
| + | <p><a href="/wiki/index.php?title=File:110901_ligation_tho_table.jpg" class="image"><img alt="110901 ligation tho table.jpg" src="/wiki/images/f/fa/110901_ligation_tho_table.jpg" width="1131" height="367" /></a> | ||
| + | </p><p><br /> | ||
| + | P, S opened Vector containing blue light promotor was ligated with RBS-LacZ insert. Due to huge insert lengths, insert:vector ratios were choosen as can be seen in ligation table. | ||
| + | </p><p><br /> | ||
| + | Ligation was conducted at 16° C in thermocycler o/n using 20 µl sample volume and 1 µl T4 DNA ligase. Examplary calculation of I/V ratio for 6x molar excess of insert (source: <a href="http://openwetware.org/wiki/DNA_Ligation" class="external free" rel="nofollow">http://openwetware.org/wiki/DNA_Ligation</a>): | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:Ratio_calculation.png" class="image"><img alt="Ratio calculation.png" src="/wiki/images/3/30/Ratio_calculation.png" width="587" height="53" /></a> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=55" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_5">Testing</span></h3> | ||
| + | <p>inoculation of two cultures with 5 µl of the glycerol stock of K322127 in DH5alpha | ||
| + | |||
| + | </p><p>the cultures were incubated at turn-off light (diode max. at 630 nm) at 37°C | ||
| + | </p><p>new transformation of K322127 (150ng/µl) 0,5 µl were used | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=56" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_6">Testing</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=57" title="Edit section: Preparing lacZ-ONPG or also called Miller Assay">edit</a>]</span> <span class="mw-headline" id="Preparing_lacZ-ONPG_or_also_called_Miller_Assay"> Preparing lacZ-ONPG or also called Miller Assay</span></h4> | ||
| + | <p><a href="/wiki/index.php?title=File:MillerAssay.jpg" class="image"><img alt="MillerAssay.jpg" src="/wiki/images/a/ad/MillerAssay.jpg" width="419" height="285" /></a> | ||
| + | |||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=58" title="Edit section: Other Work">edit</a>]</span> <span class="mw-headline" id="Other_Work_4">Other Work</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=59" title="Edit section: Part design in the registry">edit</a>]</span> <span class="mw-headline" id="Part_design_in_the_registry">Part design in the registry</span></h4> | ||
| + | <p>edit the general information of the part : | ||
| + | <a href="http://partsregistry.org/wiki/index.php?title=Part:BBa_K568000&action=edit" class="external free" rel="nofollow">http://partsregistry.org/wiki/index.php?title=Part:BBa_K568000&action=edit</a> | ||
| + | |||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=60" title="Edit section: others">edit</a>]</span> <span class="mw-headline" id="others">others</span></h4> | ||
| + | <p>the primer for the sequencing of reporter plasmid clone 1-5 from yesterday is sent to GATC | ||
| + | </p><p>Lars Mitschke kindly provided the E. coli strain BL21 (DE3), this strain contains a T7 Polymerase which can be induced with IPTG. | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=61" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_9">Results</span></h3> | ||
| + | <p><i>31-08-2011</i> | ||
| + | |||
| + | </p><p><b>transformations</b> | ||
| + | </p><p>no colonies on the plates for all ligations | ||
| + | </p><p><b>testing of K322127</b> | ||
| + | </p><p>the plate that was incubated under light showed no coloring but the plate became intransparent which indicates that the bacteria grew | ||
| + | </p><p>plates incubated under turn-off light (diode max. 630 nm) became intransparent as well; this plates were used for further testing ( see testing of K322127 ) | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=62" title="Edit section: 02-09-2011">edit</a>]</span> <span class="mw-headline" id="02-09-2011">02-09-2011</span></h2> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=63" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_8">Cloning</span></h3> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=64" title="Edit section: Digest of T7 promoter">edit</a>]</span> <span class="mw-headline" id="Digest_of_T7_promoter">Digest of T7 promoter</span></h4> | ||
| + | <p><a href="/wiki/index.php?title=File:020911_digest_tho.jpg" class="image"><img alt="020911 digest tho.jpg" src="/wiki/images/f/fb/020911_digest_tho.jpg" width="482" height="548" /></a> | ||
| + | </p><p><br /> | ||
| + | Full volume of digest was loaded onto gel (50 µl digest + 10 µl 6 x Gel loading buffer) | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:020911_digest_T7prom_cut_gel.jpg" class="image"><img alt="020911 digest T7prom cut gel.jpg" src="/wiki/images/8/87/020911_digest_T7prom_cut_gel.jpg" width="726" height="959" /></a> | ||
| + | </p><p><br /> | ||
| + | Band with correct size of 3,5 kbp was cut using 365 nm illumination. Illumination time was kept as short as possible, picture was taken after cutting gel slice. | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=65" title="Edit section: Gel purification of T7 promotor">edit</a>]</span> <span class="mw-headline" id="Gel_purification_of_T7_promotor">Gel purification of T7 promotor</span></h4> | ||
| + | |||
| + | <p>Band was purified using Promega's Wizard Gel purification Kit according to manufacturer's instructions. Eluted DNA was subsequently quantified with an analytical 0.7 % agarose gel (120 V for 45 min) | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:020911_quantitative_gel_tho.jpg" class="image"><img alt="020911 quantitative gel tho.jpg" src="/wiki/images/8/80/020911_quantitative_gel_tho.jpg" width="943" height="654" /></a> | ||
| + | </p><p><br /> | ||
| + | Band in line 2 corresponds to 120 ng band of marker. Therefore concentration of purified T7 promoter is approx. 24 ng/µl. RBS_lacZ band is approx. 20 ng/µl and pSB1C3 band approx. 16 ng/µl | ||
| + | </p> | ||
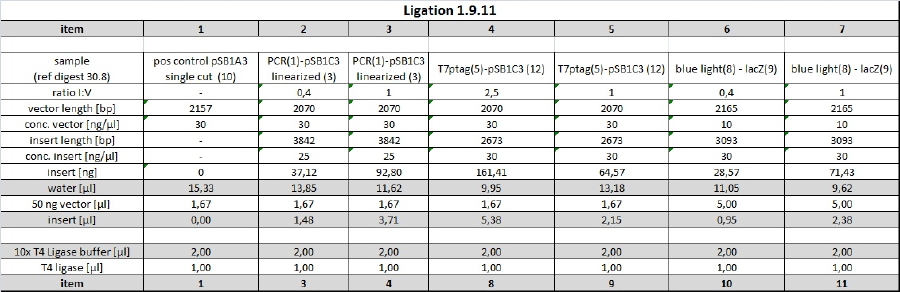
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=66" title="Edit section: Ligation of Reporter Plasmid">edit</a>]</span> <span class="mw-headline" id="Ligation_of_Reporter_Plasmid">Ligation of Reporter Plasmid</span></h4> | ||
| + | <p>digested T7 promoter was ligated with RBS_lacZ from 1.9.11 purification. | ||
| + | </p><p>Ligation was conducted o/n at 16°C. | ||
| + | </p><p><a href="/wiki/index.php?title=File:110902_ligation_tho_table.jpg" class="image"><img alt="110902 ligation tho table.jpg" src="/wiki/images/e/e7/110902_ligation_tho_table.jpg" width="1340" height="467" /></a> | ||
| + | </p> | ||
| + | |||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=67" title="Edit section: Other Work">edit</a>]</span> <span class="mw-headline" id="Other_Work_5">Other Work</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=68" title="Edit section: Agar Plates">edit</a>]</span> <span class="mw-headline" id="Agar_Plates">Agar Plates</span></h4> | ||
| + | <p>500 ml of media for ampcillin plates | ||
| + | </p><p>250 ml of media for s-gal plates | ||
| + | were prepared | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=69" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_9">Cloning</span></h4> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=70" title="Edit section: Transformation">edit</a>]</span> <span class="mw-headline" id="Transformation_2">Transformation</span></h4> | ||
| + | <p>the ligations from 01-09-2011 (1-7) were transformed into DH5alpha | ||
| + | as a transformation control 0,5 of R2 (233ng/) was transformed into DH5alpha | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=71" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_7">Testing</span></h4> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=72" title="Edit section: preparing ONPG assay">edit</a>]</span> <span class="mw-headline" id="preparing_ONPG_assay">preparing ONPG assay</span></h4> | ||
| + | |||
| + | <p>ONPG solution (4mg/ml) | ||
| + | </p><p>1 M Na2CO3 were prepared | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=73" title="Edit section: Overnight Cultures">edit</a>]</span> <span class="mw-headline" id="Overnight_Cultures">Overnight Cultures</span></h4> | ||
| + | <p>new overnight cultures of the new transformation of K322127 (see 01-09-2011) were inoculated | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=74" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_10">Results</span></h3> | ||
| + | |||
| + | <p><i>01-09-2011</i> | ||
| + | </p><p><b>Testing K322127</b> | ||
| + | </p><p>no coloring on plates | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=75" title="Edit section: 03-09-2011">edit</a>]</span> <span class="mw-headline" id="03-09-2011">03-09-2011</span></h2> | ||
| + | <p><b>People: Bea, Thorsten, Susan, Wolfgang</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=76" title="Edit section: Other work">edit</a>]</span> <span class="mw-headline" id="Other_work_6">Other work</span></h3> | ||
| + | |||
| + | <p>Cloramphenicol stock was produced: 30 mg/ml final concentration in 100% ethanol. 153 mg + 5,1 ml 100% EtOH. 500 µl aliquotes were stored at -20°C. | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=77" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_10">Cloning</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=78" title="Edit section: Precipitation of Ligations and Transformation">edit</a>]</span> <span class="mw-headline" id="Precipitation_of_Ligations_and_Transformation">Precipitation of Ligations and Transformation</span></h4> | ||
| + | <p>Todays Transformation were done after purifing ligation mix with Glycogen/Ethanol precipitation in order to increase transformed amount and purity of ligated vectors. | ||
| + | </p><p>All Ligations of 02-09-11 and following items of 01-09-11 ligation were used: | ||
| + | </p> | ||
| + | |||
| + | <dl><dd>1 (pos control pSB1A3single cut (10)) | ||
| + | </dd></dl> | ||
| + | <dl><dd>2 (PCR(1)-pSB1C3 linearized (3)) | ||
| + | </dd></dl> | ||
| + | <dl><dd>4 (T7ptag(1)-pSB1C3 (12)) | ||
| + | </dd></dl> | ||
| + | <dl><dd>6 (blue light(8) - lacZ(9)) | ||
| + | </dd></dl> | ||
| + | <p><br /> | ||
| + | Glycogen/ethanol precipitation: | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <dl><dd>-20 µl of the ligation mixed with 2 µl NaAc (3M), 0.5 µl glycogen and 22.5 µl isopropanol | ||
| + | </dd></dl> | ||
| + | <dl><dd>-incubation step for 1 h @ -20 °C | ||
| + | </dd></dl> | ||
| + | <dl><dd>-15 mins @ 10.000 rpms | ||
| + | |||
| + | </dd></dl> | ||
| + | <dl><dd>-withdraw supernatant | ||
| + | </dd></dl> | ||
| + | <dl><dd>-wash pelett with 70 % EtOH ~ 10 µl | ||
| + | </dd></dl> | ||
| + | <dl><dd>-dry pelett and resuspense in 10 µl water | ||
| + | </dd></dl> | ||
| + | <p><br /> | ||
| + | Transformation via electroporation was done with the whole cleaned 10 µl in 40 µl DH5alpha cells. | ||
| + | </p> | ||
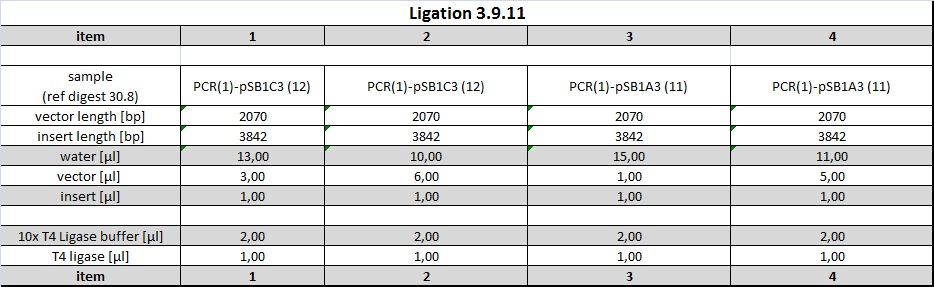
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=79" title="Edit section: Ligations of PCR product into pSB1C3 and pSB1A3">edit</a>]</span> <span class="mw-headline" id="Ligations_of_PCR_product_into_pSB1C3_and_pSB1A3">Ligations of PCR product into pSB1C3 and pSB1A3</span></h4> | ||
| + | |||
| + | <p>Following ligations were examined using an excess of vector and 1 µl PCR-product (corresponds to approx 14 ng/µl). | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:110903_ligation_susan_table.jpg" class="image"><img alt="110903 ligation susan table.jpg" src="/wiki/images/2/2e/110903_ligation_susan_table.jpg" width="934" height="287" /></a> | ||
| + | </p><p><br /> | ||
| + | Items 1+2 were incubated at room temperature and items 3+4 were incubated at 37° C for 1h each. 5 µl of ligations were subsequently electroporated into 40 µl undiluted DH5alpha cells. | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=80" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_11">Results</span></h3> | ||
| + | <p><b>02-09-2011</b> | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=81" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_8">Testing</span></h4> | ||
| + | |||
| + | <p>no coloring on plates | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=82" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_11">Cloning</span></h4> | ||
| + | <p>Single cut vector control(1) showed approx. 50 colonies. ALl ligations plated on Cam plates (2-5) showed no colonies. | ||
| + | </p><p>Blue light - RBS_lacZ ligations (6+7) showed 5 colonies overall. Clones were inoculated into 5 ml LB/Amp and incubated at 37°C o/n. | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=83" title="Edit section: 04-09-2011">edit</a>]</span> <span class="mw-headline" id="04-09-2011">04-09-2011</span></h2> | ||
| + | |||
| + | <p><b>People: Bea, Thorsten, Flo</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=84" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_12">Results</span></h3> | ||
| + | <p><b>03-09-2011</b> | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=85" title="Edit section: Transformation of purified ligations (02-09-2011)">edit</a>]</span> <span class="mw-headline" id="Transformation_of_purified_ligations_.2802-09-2011.29">Transformation of purified ligations (02-09-2011)</span></h4> | ||
| + | |||
| + | <p>positive control showed several thousand red colonies. All ligations showed bacterial lawn, including vector control. | ||
| + | </p><p><br /> | ||
| + | <span style="color:red">Vector prep contains uncut or single cut vector. This result is consistent with sequencing results, which indicate mixed clones. Next steps: Preperation of 46 bp T7 promoter insert with 3 % preparative agarose gel and ligation upstream of RBS_lacZ into opened RBS_lacZ vector. In parallel vector prep of T7 promoter will be repeated on 1% agarose gel with a long gel and run. Uncut and single cut vector will be loaded on gel aswell.</span> | ||
| + | </p> | ||
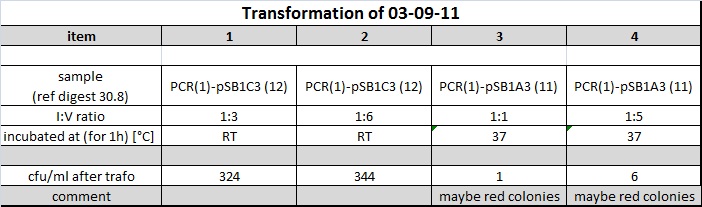
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=86" title="Edit section: Transformation of 03-09-2011 ligations">edit</a>]</span> <span class="mw-headline" id="Transformation_of_03-09-2011_ligations">Transformation of 03-09-2011 ligations</span></h4> | ||
| + | <p>showed following output: | ||
| + | </p><p><a href="/wiki/index.php?title=File:110903_trafo_susan_table.jpg" class="image"><img alt="110903 trafo susan table.jpg" src="/wiki/images/f/f8/110903_trafo_susan_table.jpg" width="702" height="207" /></a> | ||
| + | </p><p>pSB1A3 colonies were possibly red. --> incubated again o/n | ||
| + | |||
| + | </p><p><a href="http://partsregistry.org/Part:BBa_J04450" class="external free" rel="nofollow">http://partsregistry.org/Part:BBa_J04450</a> --> red color indicates religated or uncut vector clone | ||
| + | </p><p><br /> | ||
| + | 4 clones of each successful transformation were picked and inoculated into 5 ml LB medium containing the appropriate antibiotica. | ||
| + | </p><p>Conclusion: | ||
| + | </p><p>Further incubations should be done at RT for 1h or o/n at 16°C with subsequent gylkogen/ethanol purification and transformation of whole 10 µl resuspended pellet into 40 µl DH5alpha | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=87" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_12">Cloning</span></h3> | ||
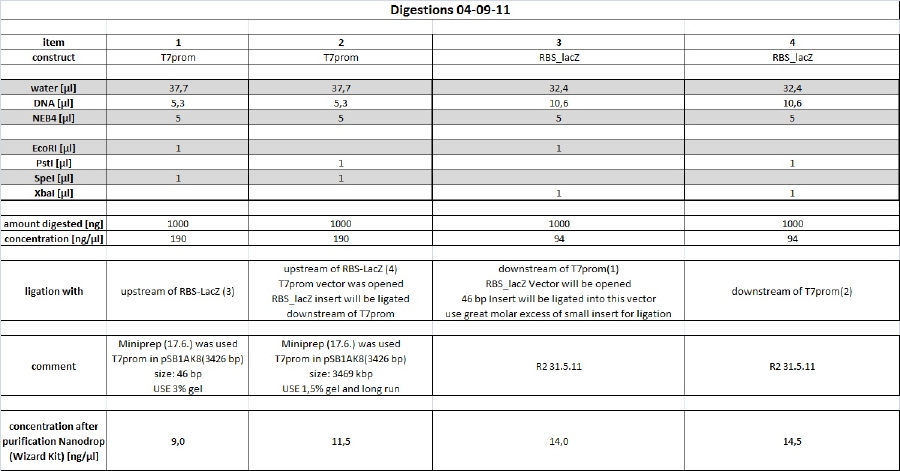
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=88" title="Edit section: Reporter Plasmid">edit</a>]</span> <span class="mw-headline" id="Reporter_Plasmid">Reporter Plasmid</span></h4> | ||
| + | |||
| + | <p>As recent transformation of reporter plasmid yielded high vector background new preps will be done according to following scheme. | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h5><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=89" title="Edit section: digest">edit</a>]</span> <span class="mw-headline" id="digest">digest</span></h5> | ||
| + | <p><a href="/wiki/index.php?title=File:110904_digest_tho.jpg" class="image"><img alt="110904 digest tho.jpg" src="/wiki/images/a/a7/110904_digest_tho.jpg" width="1199" height="628" /></a> | ||
| + | </p><p>long 1% preperative agarose gel and 3% preperative agarose gel have been produced and stored in a plastic bag with some 1x TAE buffer at 4°C o/n. Purification will be done tomorrow. | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=90" title="Edit section: Minipreps of picked clones of Bluelight lacZ 01-09-11 ligation (items 6+7)">edit</a>]</span> <span class="mw-headline" id="Minipreps_of_picked_clones_of_Bluelight_lacZ_01-09-11_ligation_.28items_6.2B7.29">Minipreps of picked clones of Bluelight_lacZ 01-09-11 ligation (items 6+7)</span></h4> | ||
| + | |||
| + | <p>[Flo] | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=91" title="Edit section: Miniprep of 5 o/n cultures [names below protocol] (Metabion Kit)">edit</a>]</span> <span class="mw-headline" id="Miniprep_of_5_o.2Fn_cultures_.5Bnames_below_protocol.5D_.28Metabion_Kit.29">Miniprep of 5 o/n cultures [names below protocol] (Metabion Kit)</span></h3> | ||
| + | <p>All centrifugation steps were performed at 13.000 rpm and RT | ||
| + | </p><p>1. Pellet 2 x 1,5 ml of bacterial suspension in <b>one</b> 2 ml-Eppi, centrifuge for 1 min each | ||
| + | </p><p>2. Add 250 ul Bact.resuspension buffer, vortex | ||
| + | </p><p>3. Add 250 ul Cell lysis buffer, invert 5x, incub. approx. 4 min | ||
| + | </p><p>4. Add 350 ul DNA binding buffer, invert 5x, centrifuge for 10 min | ||
| + | </p><p>5. Transfer clear lysate on column, centrifuge for 1 min, discard supernatant | ||
| + | |||
| + | </p><p>6. Add 600 ul Column Wash Buffer, centrifuge for 1 min | ||
| + | </p><p>7. Transfer column to new 1,5 ml-Eppi | ||
| + | </p><p>8. Add 50 ul nuclease-free water (from Promega Wizard Kit), incub. approx. 2 min, centrifuge for 1 min | ||
| + | </p><p>9. Store at -20°C | ||
| + | </p><p>names: | ||
| + | </p><p>-Blue Prom + lacZ #1 (= former "blue lacZ Trafo 7, Klon 2") | ||
| + | </p><p>-Blue Prom + lacZ #2 (= former "blue lacZ Trafo 7, Klon 1") | ||
| + | </p><p>-Blue Prom + lacZ #3 (= former "blue lacZ Trafo 6, Klon 1") | ||
| + | </p><p>-Cph8 in DH5a | ||
| + | </p><p>-K322127 | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=92" title="Edit section: Preparing glycerol stocks from cultures used above">edit</a>]</span> <span class="mw-headline" id="Preparing_glycerol_stocks_from_cultures_used_above">Preparing glycerol stocks from cultures used above</span></h4> | ||
| + | |||
| + | <p>Prepare 50% glycerol/LB solution by mixing 2 ml 100% glycerol and 2 ml LB | ||
| + | </p><p>Add 300 ul of 50% glycerol/LB solution to 700 ul bacterial suspension to obtain an end concentration of 15% glycerol | ||
| + | </p><p>Store at -80°C | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=93" title="Edit section: Restriction digest of 5 Minipreps from today">edit</a>]</span> <span class="mw-headline" id="Restriction_digest_of_5_Minipreps_from_today">Restriction digest of 5 Minipreps from today</span></h4> | ||
| + | <p>Each plasmid has been cut with EcoRI HF <b>AND</b> EcoRI HF + PstI HF (= 2 digests per plasmid) as follows: | ||
| + | </p><p>-5 ul Plasmid DNA | ||
| + | </p><p>-2 ul 10x NEB buffer 4 | ||
| + | </p><p>-0.5 ul EcoRI HF | ||
| + | |||
| + | </p><p>-(0.5 ul PstI HF) | ||
| + | </p><p>-12 (11.5) ul ddH2O | ||
| + | </p><p>end volume is <b>20 ul</b> | ||
| + | </p><p>mix, spin down, incub. at 37°C for 1 hour, inactivate at 80°C for 20 min | ||
| + | </p><p>Add 4 ul 6x loading dye | ||
| + | </p><p>Store at -20°C until tomorrow | ||
| + | </p> | ||
| + | <pre>--> quantification on gel 05-09-11 | ||
| + | </pre> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=94" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_9">Testing</span></h3> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=95" title="Edit section: ONPG-Test - Miller Assay">edit</a>]</span> <span class="mw-headline" id="ONPG-Test_-_Miller_Assay">ONPG-Test - Miller Assay</span></h4> | ||
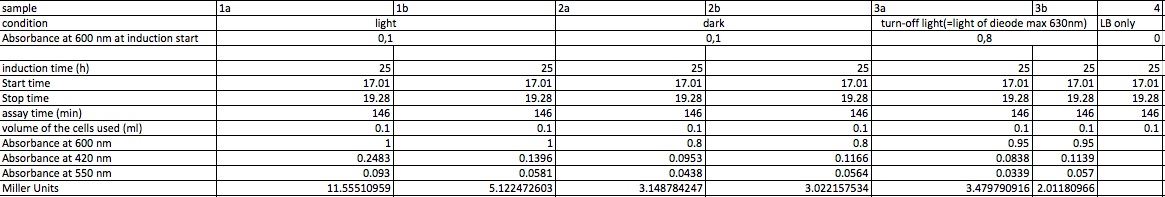
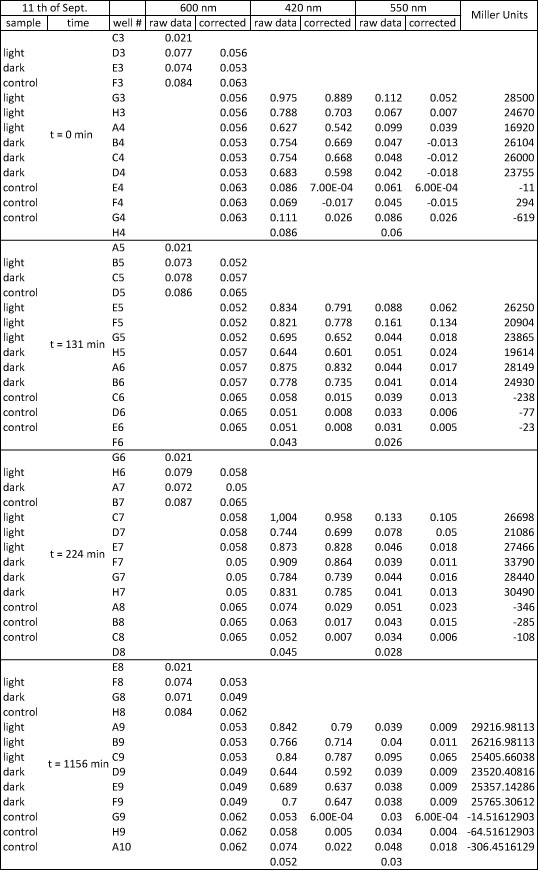
| + | <p>The assay was conducted following the protocol: | ||
| + | </p><p>3 samples of K322127 in DH5alpha were assayed: | ||
| + | 1 sample was incubated for 25 hours in the dark at room temperature | ||
| + | 1 sample was incubated for 25 hours in the light at room temperature | ||
| + | 1 sample was incubated for 25 hours in the turn-off light at room temperature | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <dl><dd>- inoculation of 4 x 2ml LB with 10 μl of overnight cultures of K322127 (see 02-09-2011) | ||
| + | </dd></dl> | ||
| + | <dl><dd>- light induction when the cultures reach OD of around | ||
| + | </dd></dl> | ||
| + | <dl><dd>- control with only LB | ||
| + | </dd></dl> | ||
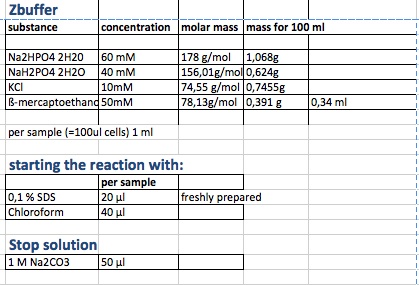
| + | <p>start of Assay: | ||
| + | |||
| + | </p> | ||
| + | <dl><dd>- measurement of Abs(600nm) | ||
| + | </dd></dl> | ||
| + | <dl><dd>- new eppi with 1 ml of Zbuffer + 20 μl of freshly prepared 0,1% SDS+ 40 μl of Chloroform (under fume hood) | ||
| + | </dd></dl> | ||
| + | <dl><dd>- 100 μl of the samples are taken and transferred to the Zbuffer | ||
| + | </dd></dl> | ||
| + | <dl><dd>- mix the solution by vortexing for 10 s | ||
| + | </dd></dl> | ||
| + | <dl><dd>- let the chloroform settle to the ground and take 700 μl of supernatant for measurement | ||
| + | </dd></dl> | ||
| + | <dl><dd>- initiation of assay with 140μl of ONPG (4mg/ml) NOTE START TIME | ||
| + | </dd></dl> | ||
| + | <dl><dd>(in the paper they use 100 μl supernatant plus 20 μl of ( 4mg/ml but we need more for measurement in cuvette) | ||
| + | </dd></dl> | ||
| + | <dl><dd>-incubation at room temperature until yellow color develops | ||
| + | </dd></dl> | ||
| + | |||
| + | <dl><dd>-stop reaction with 350 μl of 1 M Na2CO3 NOTE STOP TIME (we upscaled the paper uses 50 μl) | ||
| + | </dd></dl> | ||
| + | <dl><dd>- measurement of Abs (420nm) and Abs(550nm) | ||
| + | </dd></dl> | ||
| + | <p>Abs 420 nm should be less than 1 and more than 0,5 | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=96" title="Edit section: Result">edit</a>]</span> <span class="mw-headline" id="Result_3">Result</span></h4> | ||
| + | <p>incubation for 146 min because no visible yellow color developed; | ||
| + | </p><p>not completely clear how to calculate the miller units: | ||
| + | </p><p>here miller units are calculated as following: | ||
| + | </p><p>units= 1000* (Abs 420-1,75 Abs550) /(time(min)*Abs 600/(11,6*1,7))) | ||
| + | </p><p><br /> | ||
| + | |||
| + | <a href="/wiki/index.php?title=File:Miller_4.9.jpg" class="image"><img alt="Miller 4.9.jpg" src="/wiki/images/b/bc/Miller_4.9.jpg" width="1165" height="197" /></a> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=97" title="Edit section: Other Work">edit</a>]</span> <span class="mw-headline" id="Other_Work_7">Other Work</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=98" title="Edit section: S-Gal plates">edit</a>]</span> <span class="mw-headline" id="S-Gal_plates">S-Gal plates</span></h4> | ||
| + | <p>Flo did Minipreps of cph8 from Uppsala and K322127. | ||
| + | Nanodrop yielded no reliable data. --> quantification on gel | ||
| + | |||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=99" title="Edit section: 05-09-2011">edit</a>]</span> <span class="mw-headline" id="05-09-2011">05-09-2011</span></h2> | ||
| + | <p><b>People: Bea, Thorsten, Anna</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=100" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_10">Testing</span></h3> | ||
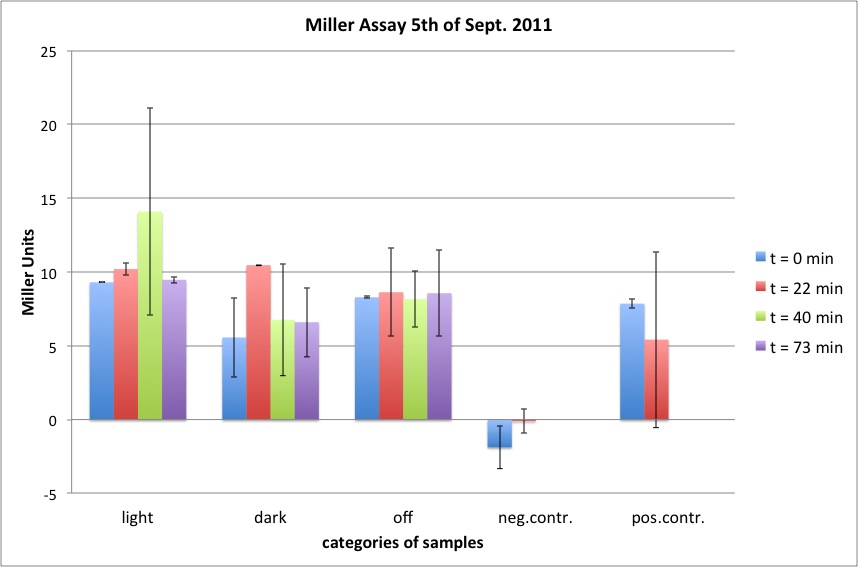
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=101" title="Edit section: Miller Assay">edit</a>]</span> <span class="mw-headline" id="Miller_Assay">Miller Assay</span></h4> | ||
| + | |||
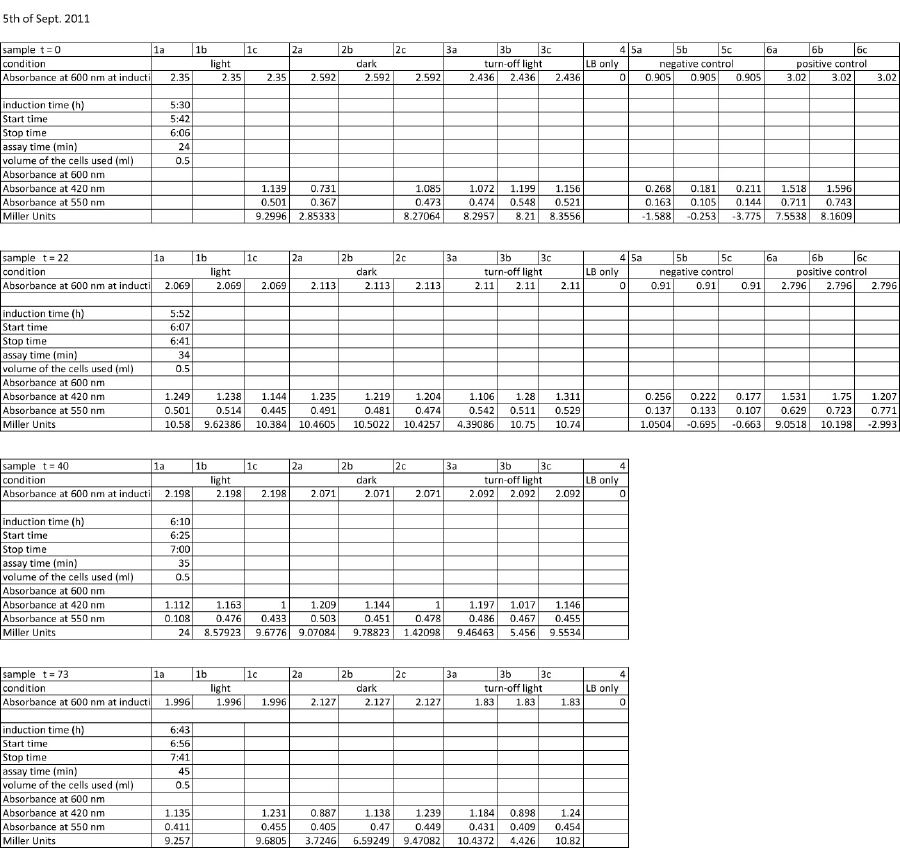
| + | <p>5 newly inoculated cultures were assayed: | ||
| + | </p> | ||
| + | <pre>after 5 hours of incubation the inducation was started and the first assay started: | ||
| + | </pre> | ||
| + | <p>induction start means: | ||
| + | incubation at room temperature of: | ||
| + | K322127 in DH5alpha in the light of lamp | ||
| + | K322127 in DH5alpha in the dark | ||
| + | K322127 in DH5alpha in the turn-off light | ||
| + | K322127 in BL21in the light | ||
| + | cpH8 in DH5 alpha in the light | ||
| + | </p><p>a new protocol was used for this assay: | ||
| + | </p><p><br /> | ||
| + | start of Assay: | ||
| + | - measurement of Abs(600nm) | ||
| + | </p><p>- new eppi with 0,5 ml of Zbuffer + 20 μl of freshly prepared 0,1% SDS+ 40 μl of Chloroform (under fume hood) | ||
| + | </p><p>- 0,5 ml of the samples are taken and transferred to the Zbuffer | ||
| + | </p><p>- mix the solution by vortexing for 10 s ( all samples equal vortexing time) | ||
| + | </p><p>- initiation of assay with 200μl of ONPG (4mg/ml) NOTE START TIME | ||
| + | </p><p>-incubation at room temperature for see table | ||
| + | </p><p>-stop reaction with 500 μl of 1 M Na2CO3 NOTE STOP TIME | ||
| + | </p><p>- measurement of Abs (420nm) and Abs(550nm) | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:Ergebnisse_Miller_Assay_table_2011-09-05.jpg" class="image"><img alt="Ergebnisse Miller Assay table 2011-09-05.jpg" src="/wiki/images/f/f6/Ergebnisse_Miller_Assay_table_2011-09-05.jpg" width="1295" height="1221" /></a> | ||
| + | |||
| + | </p><p><a href="/wiki/index.php?title=File:Ergebnisse_Miller_Assay_2011-09-05.jpg" class="image"><img alt="Ergebnisse Miller Assay 2011-09-05.jpg" src="/wiki/images/1/15/Ergebnisse_Miller_Assay_2011-09-05.jpg" width="858" height="567" /></a> | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=102" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_13">Cloning</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=103" title="Edit section: Analytical gel of Bluelight lacZ, cph8 from Uppsala and K322127 testdigest">edit</a>]</span> <span class="mw-headline" id="Analytical_gel_of_Bluelight_lacZ.2C_cph8_from_Uppsala_and_K322127_testdigest">Analytical gel of Bluelight_lacZ, cph8 from Uppsala and K322127 testdigest</span></h4> | ||
| + | <p><a href="/wiki/index.php?title=File:040911_anal_digestions_flo_tho.jpg" class="image"><img alt="040911 anal digestions flo tho.jpg" src="/wiki/images/f/f3/040911_anal_digestions_flo_tho.jpg" width="1182" height="853" /></a> | ||
| + | </p><p><br /> | ||
| + | |||
| + | Size of bands look fine. Expected: 3093bp(lacZ)+86bp(Bluelight)= 3179 bp | ||
| + | </p><p>--> Send clone 2 for sequencing or load RBS_lacZ digest along with ligated construct on gel and check for 86 bp difference. | ||
| + | </p><p>cph8(2,2kbp) and K322127(4kbp) are running on their correct sizes. | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=104" title="Edit section: Miniprep and testdigest of picked colonies on 04-09-2011">edit</a>]</span> <span class="mw-headline" id="Miniprep_and_testdigest_of_picked_colonies_on_04-09-2011">Miniprep and testdigest of picked colonies on 04-09-2011</span></h4> | ||
| + | <p>Miniprep of yesterdays picked clones were done. Concentration was determined using Nanodrop. | ||
| + | </p><p>T7 Pol in pSB1C3 (ligation 4 from 1.9.)samples: | ||
| + | </p><p>clone 1 : 58,5 ng/µl (sample 1) | ||
| + | </p><p>clone 2 : 60,5 ng/µl (sample 2) | ||
| + | |||
| + | </p><p>clone 3 : 55,5 ng/µl (sample 10) | ||
| + | </p><p>clone 4 : 56,5 ng/µl (sample 13) | ||
| + | </p><p>PCR in linearized pSB1C3: | ||
| + | </p><p>clone 1 : 44,0 ng/µl (sample 4) | ||
| + | </p><p>clone 2 : 82,0 ng/µl (sample 7) | ||
| + | </p><p>clone 3 : 77,5 ng/µl (sample 5) | ||
| + | </p><p>clone 4 : 70,0 ng/µl (sample 14) | ||
| + | </p><p>blue light promotor+ lacZ (ligation 6 from 1.9): | ||
| + | </p><p>clone 1 : 79,0 ng/µl (sample 12) | ||
| + | </p><p>clone 2 : 67,0 ng/µl (sample 9) | ||
| + | |||
| + | </p><p>clone 3 : 55,5 ng/µl (sample 21) | ||
| + | </p><p>clone 4 : 65,0 ng/µl (sample 19) | ||
| + | </p><p>red light sensor in pSB1C3 ratio 1:3 | ||
| + | </p><p>clone 1 : 58,0 ng/µl (sample 11) | ||
| + | </p><p>clone 2 : 52,5 ng/µl (sample 15) | ||
| + | </p><p>clone 3 : 59,5 ng/µl (sample 8) | ||
| + | </p><p>red light sensor in pSB1C3 ratio 1:6 | ||
| + | </p><p>clone 1 : 50,5 ng/µl (sample 18) | ||
| + | </p><p>clone 2 : 58,5 ng/µl (sample 3) | ||
| + | </p><p>clone 3 : 61,5 ng/µl (sample 6) | ||
| + | |||
| + | </p><p>red light sensor in pSB1A3 (ligation 1:5 (from 1.9.) | ||
| + | clone 1 : 52,5 ng/µl (sample 20)(red colony) | ||
| + | clone 2 : 51,0 ng/µl (sample 16) | ||
| + | </p><p>red light sensor in pSB1A3 (ligation 1:1 (from 3.9) (red colony): | ||
| + | clone 1 : 40,5 ng/µl (sample 17) | ||
| + | </p><p>All samples were digested except 9,21,19: | ||
| + | </p><p>5 µl of Mini-DNA was cut using 0,5 µl E and 0,5 µl P in 2 µl NEB4 and 20 µl total volume. Digest was loaded onto 1 % agarose gel. | ||
| + | </p><p><a href="/wiki/index.php?title=File:040911_anal_ligations.jpg" class="image"><img alt="040911 anal ligations.jpg" src="/wiki/images/7/7c/040911_anal_ligations.jpg" width="1033" height="709" /></a> | ||
| + | </p><p><br /> | ||
| + | Red light sensor constructs look fine (3,8kbp insert and 2 kbp vector). T7ptag constructs are wrong and will be repeated (T7ptag expected at 2,6 kbp). Blue light sensor looks fine (3kbp). Red light sensor cloning into pSB1A3 didnt yield any insertion products, mainly slightly red colonies were visible --> 1kbp insert is RFP cassette. One not-red clone could be picked (sample 15), which contains a 400 bp insert of unkown origin. | ||
| + | </p><p>--> Sample 7 was choosen to be sequenced as it yielded most amount of DNA. | ||
| + | </p> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=105" title="Edit section: Reporter Plasmid: T7 promotor prep and new RBS LacZ prep">edit</a>]</span> <span class="mw-headline" id="Reporter_Plasmid:_T7_promotor_prep_and_new_RBS_LacZ_prep">Reporter Plasmid: T7 promotor prep and new RBS_LacZ prep</span></h4> | ||
| + | <p>3 % agarose gel from yesterday was used at 100V for 1,5h | ||
| + | </p><p><a href="/wiki/index.php?title=File:040911_3percentgel_tho.jpg" class="image"><img alt="040911 3percentgel tho.jpg" src="/wiki/images/1/12/040911_3percentgel_tho.jpg" width="707" height="993" /></a> | ||
| + | </p><p><br /> | ||
| + | No band could be detected. Nevertheless, gel slice was cut and will be purified. | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:040911_t7prom_cut_tho.jpg" class="image"><img alt="040911 t7prom cut tho.jpg" src="/wiki/images/9/95/040911_t7prom_cut_tho.jpg" width="633" height="1002" /></a> | ||
| + | </p><p><br /> | ||
| + | Bands were cut and purified using Wizard Kit according to manufacturer's instructions. | ||
| + | </p><p>Concentrations was determined using Nanodrop: | ||
| + | </p><p>T7prom E, S: 9 ng/µl | ||
| + | |||
| + | </p><p>T7prom P, S: 11.5 ng/µl | ||
| + | </p><p>RBS_lacZ E, X: 14 ng/µl | ||
| + | </p><p>RBS_lacZ E, X: 14.5 ng/µl | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=106" title="Edit section: 06-09-2011">edit</a>]</span> <span class="mw-headline" id="06-09-2011">06-09-2011</span></h2> | ||
| + | <p><b>People: Thorsten, Anna, Katharina</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=107" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_11">Testing</span></h3> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=108" title="Edit section: Miller Assay">edit</a>]</span> <span class="mw-headline" id="Miller_Assay_2">Miller Assay</span></h4> | ||
| + | <p>5 newly inoculated cultures were assayed: | ||
| + | difference to day before: grow in the dark (only DH5alpha) | ||
| + | </p><p>incubation at room temperature of: | ||
| + | </p><p>K322127 in DH5alpha in the light of lamp | ||
| + | </p><p>K322127 in DH5alpha in the dark | ||
| + | </p><p>K322127 in DH5alpha in the turn-off light | ||
| + | </p><p>K322127 in BL21 in the light | ||
| + | </p><p>cpH8 in DH5 alpha in the light | ||
| + | </p><p>Problem 1: OD of bacterial cultures was very high, should have been around 0,7. | ||
| + | </p><p>Problem 2: too long incubation of color reaction yielded too high ODs, samples couldn't be diluted as more blank sample (as diluent) was missing. | ||
| + | </p><p><a href="/wiki/index.php?title=File:Miller_Assay_Tabelle_2011-09-06.jpg" class="image"><img alt="Miller Assay Tabelle 2011-09-06.jpg" src="/wiki/images/7/74/Miller_Assay_Tabelle_2011-09-06.jpg" width="1295" height="1701" /></a> | ||
| + | </p><p><a href="/wiki/index.php?title=File:Miller_Assay_2011-09-06.jpg" class="image"><img alt="Miller Assay 2011-09-06.jpg" src="/wiki/images/7/78/Miller_Assay_2011-09-06.jpg" width="895" height="599" /></a> | ||
| + | |||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=109" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_14">Cloning</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=110" title="Edit section: Reporter Plasmid">edit</a>]</span> <span class="mw-headline" id="Reporter_Plasmid_2">Reporter Plasmid</span></h4> | ||
| + | <h5><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=111" title="Edit section: Purification of 05-09-11 gel runs">edit</a>]</span> <span class="mw-headline" id="Purification_of_05-09-11_gel_runs">Purification of 05-09-11 gel runs</span></h5> | ||
| + | |||
| + | <p>Frozen gel slices were purified using Promega's Wizard Kit according to manufacturers instructions. | ||
| + | </p><p>Subsequent determination of concentration via Nanodrop: | ||
| + | </p><p>1: T7prom 46 bp cut: 9 ng/µl | ||
| + | </p><p>2: T7prom vector cut: 11.5 ng/µl | ||
| + | </p><p>3: RBS_lacZ vector cut: 14.0 ng/µl | ||
| + | </p><p>4: RBS_lacZ insert cut: 14.5 ng/µl | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h5><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=112" title="Edit section: Ligation of purified samples: Reporter Plasmid and T7ptag into shipping vector pSB1C3">edit</a>]</span> <span class="mw-headline" id="Ligation_of_purified_samples:_Reporter_Plasmid_and_T7ptag_into_shipping_vector_pSB1C3">Ligation of purified samples: Reporter Plasmid and T7ptag into shipping vector pSB1C3</span></h5> | ||
| + | <p><a href="/wiki/index.php?title=File:110906_digest_tho.jpg" class="image"><img alt="110906 digest tho.jpg" src="/wiki/images/b/be/110906_digest_tho.jpg" width="1868" height="539" /></a> | ||
| + | </p><p><br /> | ||
| + | Ligation mix was incubated for 1h at RT and and then at 16°C o/n. | ||
| + | |||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=113" title="Edit section: Other Work">edit</a>]</span> <span class="mw-headline" id="Other_Work_8">Other Work</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=114" title="Edit section: Sequencing of clones">edit</a>]</span> <span class="mw-headline" id="Sequencing_of_clones">Sequencing of clones</span></h4> | ||
| + | <p>Primer overview: <a href="http://partsregistry.org/Primers/Catalog" class="external free" rel="nofollow">http://partsregistry.org/Primers/Catalog</a> | ||
| + | </p><p><br /> | ||
| + | |||
| + | For most biobrick plasmids the standard sequencing primers VF2 (verification forward) and VR (verification reverse) will fit. They will be synthesized at GATC and stored for 1 year. | ||
| + | </p><p><br /> | ||
| + | 10 µl of Following clones were send for sequencing 300-100 ng/µl with VF2 primer (tgccacctgacgtctaagaa): | ||
| + | </p><p><br /> | ||
| + | Bluelight_lacZ in pSB1A2 110904_clone2 miniprep conc.: 49.5 ng/µl | ||
| + | </p><p><br /> | ||
| + | red light in pSB1C3 110905_sample 7 conc.: 82 ng/µl diluted 1:1 with ddH2O | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=115" title="Edit section: 07-09-2011">edit</a>]</span> <span class="mw-headline" id="07-09-2011">07-09-2011</span></h2> | ||
| + | <p><b>People: Thorsten, Anna, Katharina</b> | ||
| + | </p> | ||
| + | |||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=116" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_15">Cloning</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=117" title="Edit section: Reporter Plasmid and T7ptag into pSB1C3">edit</a>]</span> <span class="mw-headline" id="Reporter_Plasmid_and_T7ptag_into_pSB1C3">Reporter Plasmid and T7ptag into pSB1C3</span></h4> | ||
| + | <h5><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=118" title="Edit section: Glycogen/ethanol precipitation and transformation">edit</a>]</span> <span class="mw-headline" id="Glycogen.2Fethanol_precipitation_and_transformation">Glycogen/ethanol precipitation and transformation</span></h5> | ||
| + | |||
| + | <p>- DNA precipitation with glycogen:of the ligation samples from 6-9-11 | ||
| + | </p><p>1. add 2 µl 3 M sodium acetate, 0.5 µl glycogen and 22.5 µl isopropanol to 20 µl ligation product | ||
| + | </p><p>2. incubate the mixture at -20°C for one hour | ||
| + | </p><p>3. centrifuge the mixture for 15 minutes at 18,000 rpm, 4°C | ||
| + | </p><p>4. discard the supernatant | ||
| + | </p><p>5. add 50 µl 70 % ethanol | ||
| + | </p><p>6. centrifuge the mixture for another 10 minutes at 18,000 rpm, 4°C | ||
| + | </p><p>7. remove supernatant with a pipet CAREFULLY. If pellet moves, centrifuge again. | ||
| + | </p><p>8. air-dry the pellet until the ethanol is completely evaporated. Do not over-dry pellet as this makes dissolving more difficult. | ||
| + | </p><p>9. resuspend the pellet in 10 µl nuclease-free water by pipetting carefully. | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <ul><li>10 µl purified ligation was mixed with 40 µl competent cells and electroporated into DH5alpha. | ||
| + | </li><li>1 ml SOC medium (SOB medium + 20 mM glucose) was used and incubation was carried out for 1h at 37°C, shaking at 500 rpm in 2 ml eppis. | ||
| + | </li><li>Culture was then centrifuged at 4000 rpm for 5 minutes, and resolved in 100 µl medium. | ||
| + | </li><li>Whole sample was plated onto LB/agar plates supplemented with appropriate antibiotic and incubated o/n @ 37°C | ||
| + | |||
| + | </li></ul> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=119" title="Edit section: Synthetic construct">edit</a>]</span> <span class="mw-headline" id="Synthetic_construct">Synthetic construct</span></h4> | ||
| + | <h5><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=120" title="Edit section: Digest of synthetic construct and PCR-product (in pSB1C3)">edit</a>]</span> <span class="mw-headline" id="Digest_of_synthetic_construct_and_PCR-product_.28in_pSB1C3.29">Digest of synthetic construct and PCR-product (in pSB1C3)</span></h5> | ||
| + | <p><a href="/wiki/index.php?title=File:110907_digest_tho_.jpg" class="image" title="110907 digest tho .jpg"><img alt="110907 digest tho .jpg" src="/wiki/images/4/4a/110907_digest_tho_.jpg" width="796" height="448" /></a> | ||
| + | </p><p><br /> | ||
| + | Samples were loaded on preparative gel (1 %, TAE 1x, 100 V, 2 h 30 min) and subsequently cut with a new scalpel for each sample using 365 nm illumination as short as possible. Gel slices were purified using Promega's Wizard Kit according to manufacturer's instructions. Picture was taken after cutting of gel slices in order to reduce illumination. | ||
| + | |||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:7911-preparative-gel-red-light-synthetisch.jpg" class="image"><img alt="7911-preparative-gel-red-light-synthetisch.jpg" src="/wiki/images/2/21/7911-preparative-gel-red-light-synthetisch.jpg" width="1006" height="975" /></a> | ||
| + | </p> | ||
| + | <h5><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=121" title="Edit section: ligation of synthetic construct and PCR-product (in pSB1C3)">edit</a>]</span> <span class="mw-headline" id="ligation_of_synthetic_construct_and_PCR-product_.28in_pSB1C3.29">ligation of synthetic construct and PCR-product (in pSB1C3)</span></h5> | ||
| + | <p><a href="/wiki/index.php?title=File:110907_ligation.jpg" class="image"><img alt="110907 ligation.jpg" src="/wiki/images/d/df/110907_ligation.jpg" width="1462" height="458" /></a> | ||
| + | </p><p><br /> | ||
| + | Ligation was performed at 16°C o/n | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=122" title="Edit section: 08-09-2011">edit</a>]</span> <span class="mw-headline" id="08-09-2011">08-09-2011</span></h2> | ||
| + | |||
| + | <p><b>People: Anna, Katharina</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=123" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_16">Cloning</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=124" title="Edit section: AND-Gate plasmid">edit</a>]</span> <span class="mw-headline" id="AND-Gate_plasmid">AND-Gate plasmid</span></h4> | ||
| + | <p>- DNA precipitation with glycogen/ethanol of the ligation samples from 7-9-11 accroding to protocol 7-9-11 | ||
| + | </p><p><br /> | ||
| + | |||
| + | - transformation via electroporation of 40 µl electro competent DH5alpha cells with the full volume of purified ligation samples from 7-9-11 | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=125" title="Edit section: Reporter plasmid and T7ptag">edit</a>]</span> <span class="mw-headline" id="Reporter_plasmid_and_T7ptag">Reporter plasmid and T7ptag</span></h4> | ||
| + | <p>-overnight cultures for mini prep of transformations from 7-9-2011: | ||
| + | </p><p>four clones of plates: | ||
| + | </p><p>2 (T7prom(2)_RBS_lacZ(4)) | ||
| + | </p><p>3 (T7prom(2)_RBS_lacZ(4)) | ||
| + | </p><p>5 (RBS_lacZ(3)_T7prom(1)) | ||
| + | </p><p>6 (pSB1C3(3)_T7pol(5)) | ||
| + | </p><p>7 (pSB1C3(3)_T7pol(5)) | ||
| + | </p><p><br /> | ||
| + | one clone of plate 13 (synthetic construct in delivery vector pMA-RQ) | ||
| + | |||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=126" title="Edit section: Other work">edit</a>]</span> <span class="mw-headline" id="Other_work_9">Other work</span></h3> | ||
| + | <p>- production of LB-Agar plates with Kanamycin (500 ml), Chloramphenicol (300 ml) and Ampicillin (500 ml) | ||
| + | </p><p>- overnight cultures of K322127 in DH5alpha (Cam, in the dark) and cpH8 inDH5alpha (Kan) for Miller Assay the next day | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=127" title="Edit section: 09-09-2011">edit</a>]</span> <span class="mw-headline" id="09-09-2011">09-09-2011</span></h2> | ||
| + | |||
| + | <p><b>People: Anna, Katharina, Thorsten</b> | ||
| + | </p><p>Std Primer VR seems to misprime to B0015 double terminator: <a href="http://partsregistry.org/Help:Primers/Problems_using_VF2_and_VR_to_amplify_BioBrick_parts" class="external free" rel="nofollow">http://partsregistry.org/Help:Primers/Problems_using_VF2_and_VR_to_amplify_BioBrick_parts</a> | ||
| + | </p><p>Could be a problem when sequencing into our construct from downstream primer binding site as we use B0015 in synthetic construct. Because sequencing was done succesfully using this primer by others, we'll try it keeping the mispriming in mind. --> B0014 would be a better terminator for next time. | ||
| + | </p><p>pSB1C3 is using B0056 as terminator downstream of suffix. | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=128" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_17">Cloning</span></h3> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=129" title="Edit section: Reporter plasmid and T7 polymerase">edit</a>]</span> <span class="mw-headline" id="Reporter_plasmid_and_T7_polymerase">Reporter plasmid and T7 polymerase</span></h4> | ||
| + | <p>- Plasmid isolation -> Miniprep of the over night cultures from 8-9-11 according to manufacturers instructions from Metabion | ||
| + | </p><p><br /> | ||
| + | - measurement of the DNA concentration of the following samples: | ||
| + | </p><p><br /> | ||
| + | 2a: 92 ng/µl | ||
| + | </p><p>2b: 122 ng/µl | ||
| + | </p><p>2c: 131 ng/µl | ||
| + | </p><p>3a: 98 ng/µl | ||
| + | </p><p>3b: 152 ng/µl | ||
| + | |||
| + | </p><p>3c: 124 ng/µl | ||
| + | </p><p>3d: 112 ng/µl | ||
| + | </p><p>5a: 52.5 ng/µl | ||
| + | </p><p>5b: 55 ng/µl | ||
| + | </p><p>5c: 74.5 ng/µl | ||
| + | </p><p>5d: 87 ng/µl | ||
| + | </p><p>6a: 134 ng/µl | ||
| + | </p><p>6b: 118 ng/µl | ||
| + | </p><p>6c: 101 ng/µl | ||
| + | </p><p>6d: 140 ng/µl | ||
| + | </p><p>7a: 76.5 ng/µl | ||
| + | </p><p>7b: 113 ng/µl | ||
| + | </p><p>7c: 83 ng/µl | ||
| + | </p><p>7d: 71.5 ng/µl | ||
| + | </p><p>13: 79 ng/µl | ||
| + | </p><p><br /> | ||
| + | - control restriction digest of the 21 DNA samples (2a-d, 3a-d, 5a-d, 6a-d, 7a-d,13) with the restricition enzymes E,P | ||
| + | |||
| + | </p><p>total volume 50 µl (39 µl nf H20, 5 µl DNA, 5 µl NEB4 Buffer, 0,5 µl of each enzyme) | ||
| + | </p><p>incubation for 1 hour at 37°C, inactivation for 20 min at 80°C | ||
| + | </p><p><br /> | ||
| + | - analytical gelelectrophoresis ( 1%, 1x TAE, 12 wells, 120 V, 1 hour 30 minutes) | ||
| + | </p><p>50 µl DNA + 10 µl loading buffer -> 40 µl sample in each well | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:110909_analytical_gel_ligation_of_110906.jpg" class="image"><img alt="110909 analytical gel ligation of 110906.jpg" src="/wiki/images/a/a2/110909_analytical_gel_ligation_of_110906.jpg" width="1338" height="864" /></a> | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:110909_analytical_gel_ligation_of_110906_part2.jpg" class="image"><img alt="110909 analytical gel ligation of 110906 part2.jpg" src="/wiki/images/b/b9/110909_analytical_gel_ligation_of_110906_part2.jpg" width="1214" height="1024" /></a> | ||
| + | </p><p><br /> | ||
| + | Size of T7promotor with RBS_lacZ looks correct (RBS_lacZ approx 3kbp pSB1AK8 approx 3.4 kbp). Ligation of 46 bp T7promotor into pSB1A2_RBS_lacZ (ID 5) didnt yield any correct clones, only vector background could be picked. Samples were discarded. Transformation of sample 4 resulted in short-circuit and was lost. Ligation of T7ptag into pSB1C3 looks fine. (T7ptag approx 2.6 kbp) | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=130" title="Edit section: AND-Gate plasmid">edit</a>]</span> <span class="mw-headline" id="AND-Gate_plasmid_2">AND-Gate plasmid</span></h4> | ||
| + | |||
| + | <p>transformation output: vector background looks fine | ||
| + | </p><p>following clones have been picked: | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=131" title="Edit section: Next steps">edit</a>]</span> <span class="mw-headline" id="Next_steps">Next steps</span></h3> | ||
| + | <p>Besides ligation of T7ptag insert, pSB1C3_T7ptag vector will be opened to ligate with red light_synthetic construct insert--> see 10-09-11. | ||
| + | </p><p>Send clones for sequencing on Monday. | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=132" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_12">Testing</span></h3> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=133" title="Edit section: Miller Assay">edit</a>]</span> <span class="mw-headline" id="Miller_Assay_3">Miller Assay</span></h4> | ||
| + | <p>two cultures in LB medium: | ||
| + | </p><p><b>K322127 in DH5alpha</b> with three incubation conditions: <b>lighted, dark and under off-light</b> | ||
| + | </p><p>cpH8 in DH5 alpha as a control | ||
| + | </p><p>conditions of bacterial growth: 37 °C, shaken, culture K322127 in DH5alpha <b>grown in the dark</b> prior to the experiment | ||
| + | |||
| + | </p><p>volume of bacterial suspension used: <b>500 µl</b> | ||
| + | </p><p>incubation time for Miller Assay: <b>10 min</b> (37 °C) | ||
| + | </p><p><br /> | ||
| + | improvement to prior experiments: start OD between 0,15 and 0,17, correct antibiotics applied, incubation time of Miller Assay only 10 min. | ||
| + | </p><p><br /> | ||
| + | </p><p><a href="/wiki/index.php?title=File:Miller_Assay_tabelle_9.9.2011.jpg" class="image"><img alt="Miller Assay tabelle 9.9.2011.jpg" src="/wiki/images/5/56/Miller_Assay_tabelle_9.9.2011.jpg" width="554" height="1111" /></a> | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:Miller_Assay_diagramm_9.9.2011.jpg" class="image"><img alt="Miller Assay diagramm 9.9.2011.jpg" src="/wiki/images/9/9f/Miller_Assay_diagramm_9.9.2011.jpg" width="853" height="508" /></a> | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=134" title="Edit section: 10-09-2011">edit</a>]</span> <span class="mw-headline" id="10-09-2011">10-09-2011</span></h2> | ||
| + | |||
| + | <p><b>People: Susan, Wolfgang, Thorsten</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=135" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_18">Cloning</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=136" title="Edit section: AND-Gate plasmid">edit</a>]</span> <span class="mw-headline" id="AND-Gate_plasmid_3">AND-Gate plasmid</span></h4> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=137" title="Edit section: red light sensor - synthetic construct ligation check">edit</a>]</span> <span class="mw-headline" id="red_light_sensor_-_synthetic_construct_ligation_check">red_light sensor - synthetic construct ligation check</span></h4> | ||
| + | |||
| + | <p>- Plasmid isolation -> Miniprep of the over night cultures from 09-09-11 according to manufacturers instructions from Metabion. 4 ml culture was used and 1 ml culture was saved for potential glycerol stocks | ||
| + | </p><p><br /> | ||
| + | - control restriction digest of the 21 DNA samples (2a-d, 3a-d, 5a-d, 6a-d, 7a-d,13) with the restricition enzymes E,S | ||
| + | </p><p>total volume 10 µl (7 µl nf H20, 1 µl DNA, 1 µl NEB4 Buffer, 1 µl of each enzyme) | ||
| + | </p><p>nuke for 1 minute at 800 W (microwave) | ||
| + | </p><p><br /> | ||
| + | </p><p><br /> | ||
| + | </p><p>- analytical gelelectrophoresis ( 0.7%, 1 x TAE, 12 wells, 160 V, 20 minutes) | ||
| + | </p><p>10 µl DNA + 2 µl loading buffer -> 12 µl sample in each well | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:110910_anal_digest_of_110907_ligations.jpg" class="image"><img alt="110910 anal digest of 110907 ligations.jpg" src="/wiki/images/6/65/110910_anal_digest_of_110907_ligations.jpg" width="995" height="628" /></a> | ||
| + | </p><p><br /> | ||
| + | Sample ID 3c was choosen to be used for further cloning. pSB1C3 with red light sensor and synthetic construct | ||
| + | |||
| + | </p><p>Nanodrop derived DNA conc. of 3c: 114 ng/µl | ||
| + | </p><p>samples 2, 3a, 4b and 6a were discarded | ||
| + | </p><p>sample 7b was choosen to be synthetic construct in pSB1C3 --> sequencing on monday | ||
| + | </p> | ||
| + | <h5><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=138" title="Edit section: last step: assembly of redlight sensor synthetic construct in pSB1C3 with T7ptag">edit</a>]</span> <span class="mw-headline" id="last_step:_assembly_of_redlight_sensor_synthetic_construct_in_pSB1C3_with_T7ptag">last step: assembly of redlight sensor_synthetic construct in pSB1C3 with T7ptag</span></h5> | ||
| + | <p>we took 2 approaches: ligation of T7ptag as insert and vector, respectively | ||
| + | </p><p><br /> | ||
| + | </p> | ||
| + | <h5><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=139" title="Edit section: Digestions">edit</a>]</span> <span class="mw-headline" id="Digestions">Digestions</span></h5> | ||
| + | |||
| + | <p><a href="/wiki/index.php?title=File:1109010_digest_tho.jpg" class="image"><img alt="1109010 digest tho.jpg" src="/wiki/images/7/76/1109010_digest_tho.jpg" width="944" height="488" /></a> | ||
| + | </p><p><br /> | ||
| + | samples were loaded on 1 % preparative agarose gel and run for 1.5 h at 120V | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:110910_prep_digest_tho.jpg" class="image"><img alt="110910 prep digest tho.jpg" src="/wiki/images/0/07/110910_prep_digest_tho.jpg" width="1231" height="922" /></a> | ||
| + | </p><p><br /> | ||
| + | Samples were cut (365 nm illumination as short as possible, picture taken after cutting of gel slices) and purified using Promegas Wizard purification kit according to manufacturers instructions. | ||
| + | </p> | ||
| + | <h5><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=140" title="Edit section: Ligations">edit</a>]</span> <span class="mw-headline" id="Ligations">Ligations</span></h5> | ||
| + | <p><a href="/wiki/index.php?title=File:110910_ligation_tho_table.jpg" class="image"><img alt="110910 ligation tho table.jpg" src="/wiki/images/8/80/110910_ligation_tho_table.jpg" width="1707" height="458" /></a> | ||
| + | </p><p>Samples were incubated o/n at 16°C. | ||
| + | |||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=141" title="Edit section: 11-09-2011">edit</a>]</span> <span class="mw-headline" id="11-09-2011">11-09-2011</span></h2> | ||
| + | <p><b>People: Katharina, Flo, Anna</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=142" title="Edit section: Glycogen/EtOH-precipitation of ligation samples from 10-09-11 and transformation">edit</a>]</span> <span class="mw-headline" id="Glycogen.2FEtOH-precipitation_of_ligation_samples_from_10-09-11_and_transformation">Glycogen/EtOH-precipitation of ligation samples from 10-09-11 and transformation</span></h3> | ||
| + | <p>Put everything on ice before starting to work! Pre-cool centrifuge! | ||
| + | |||
| + | </p><p>See protocol for precipitation/transformation from 07-09-11[<a href="http://igem.ph.tum.de/wiki/index.php?title=Labbook_Part_3#Glycogen.2Fethanol_precipitation_and_transformation" class="external autonumber" rel="nofollow">[1]</a>] | ||
| + | </p><p>Changes made: | ||
| + | </p> | ||
| + | <ul><li>all solutions were icecold | ||
| + | </li><li>pellet was air-dryed for about 10 min | ||
| + | </li><li>autoclaved water was used instead of nuclease-free, due to quick subsequent use in the transformation | ||
| + | </li><li>resuspending of the bacterial pellet after transformation was difficult (tried pipetting and vortexing), because the wrong centrifuge has been used | ||
| + | </li><li>1,5 ml-Eppis were used instead of 2 ml-Eppis for the shaking step at 37°C in SOC | ||
| + | </li></ul> | ||
| + | <p><b>Samples 1, 8 and 9</b>: comp. cells used were DH5a from <b>01-07-11</b> | ||
| + | </p><p><b>Samples 2 - 7</b>: comp. cells used were DH5a from <b>30-08-11</b> | ||
| + | |||
| + | </p><p>Antibiotic resistance: | ||
| + | </p> | ||
| + | <ul><li>1: Amp | ||
| + | </li><li>2 - 9: Cam | ||
| + | </li></ul> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=143" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_13">Testing</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=144" title="Edit section: Inoculation of o/n-cultures for tomorrow´s Miller Assay">edit</a>]</span> <span class="mw-headline" id="Inoculation_of_o.2Fn-cultures_for_tomorrow.C2.B4s_Miller_Assay">Inoculation of o/n-cultures for tomorrow´s Miller Assay</span></h4> | ||
| + | |||
| + | <p>10 ml LB+antibiotic in 50 ml-falcon tube | ||
| + | </p><p>add 10 ul (= 1:1000 dilution) of yesterday´s o/n-cultures: | ||
| + | </p> | ||
| + | <ul><li>bluelight + lacZ in DH5a (Amp) | ||
| + | </li><li>redlight + lacZ (trunc.) in DH5a (Cam) | ||
| + | </li><li>neg. contr. Cph8 in DH5a (Kan) | ||
| + | </li></ul> | ||
| + | <p><b>Wrap falcons with aluminium foil, so growth can occur in the dark!</b> | ||
| + | </p><p>Shake o/n @ 37°C, 180 rpm | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=145" title="Edit section: Miller Assay with blue light construct">edit</a>]</span> <span class="mw-headline" id="Miller_Assay_with_blue_light_construct">Miller Assay with blue light construct</span></h4> | ||
| + | |||
| + | <p>two cultures in <b>M9</b> medium: | ||
| + | </p><p><b>blue light - lacZ on pSB1A2 in DH5alpha</b> with two incubation conditions: <b>lighted in blue light and dark</b> | ||
| + | </p><p>cpH8 in DH5 alpha as a control under room light | ||
| + | </p><p>conditions of bacterial growth: 23,5 °C, shaken, both cultures grown under room light prior to the experiment | ||
| + | </p><p>volume of bacterial suspension used: <b>0,1 ml</b> | ||
| + | </p><p>incubation time for Miller Assay: 5 min (37 °C) | ||
| + | </p><p>improvement to prior experiments: start OD between 0,15 and 0,17, correct antibiotics applied | ||
| + | </p><p><br /> | ||
| + | |||
| + | Start of Assay: | ||
| + | - measurement of Abs(600nm) | ||
| + | </p><p>- new eppi with 0,5 ml of Zbuffer + 20 μl of freshly prepared 0,1% SDS+ 40 μl of Chloroform (under fume hood) | ||
| + | </p><p>- 0,1 ml of the samples are taken and transferred to the Zbuffer | ||
| + | </p><p>- mix the solution by vortexing for 10 s ( all samples equal vortexing time) | ||
| + | </p><p>- transferring of 100 μl supernatant to 96 well plate (for photometer) | ||
| + | </p><p>- initiation of assay with 20 μl of ONPG (4mg/ml) NOTE START TIME | ||
| + | </p><p>- incubation at 37 °C | ||
| + | </p><p>- stop reaction with 50 μl of 1 M Na2CO3 NOTE STOP TIME | ||
| + | </p><p>- measurement of Abs (420nm) and Abs (550nm) | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=146" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_13">Results</span></h3> | ||
| + | <p><a href="/wiki/index.php?title=File:Miller_Assay_tabelle_11.9.2011.jpg" class="image"><img alt="Miller Assay tabelle 11.9.2011.jpg" src="/wiki/images/c/c4/Miller_Assay_tabelle_11.9.2011.jpg" width="538" height="871" /></a> | ||
| + | |||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:Miller_Assay_diagramm_11.9.2011.jpg" class="image"><img alt="Miller Assay diagramm 11.9.2011.jpg" src="/wiki/images/4/49/Miller_Assay_diagramm_11.9.2011.jpg" width="709" height="449" /></a> | ||
| + | </p><p>Problems: | ||
| + | 1. cells had a party over night: three LED (blue on, red on, red off) were on alternatively | ||
| + | 2. did cells really grow? | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=147" title="Edit section: 12-09-2011">edit</a>]</span> <span class="mw-headline" id="12-09-2011">12-09-2011</span></h2> | ||
| + | <p><b>People: Thorsten, Anna</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=148" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_19">Cloning</span></h3> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=149" title="Edit section: AND-Gate: Picking of colonies">edit</a>]</span> <span class="mw-headline" id="AND-Gate:_Picking_of_colonies">AND-Gate: Picking of colonies</span></h4> | ||
| + | <p>Transformation and ligation efficiency of control was several thousand clones. Vector controls were lawn due to usage of CAM-plasmid contaminated electorcompetent DH5 alpha. All contaminated DH5alpha from 01-07-11 have been discarded. | ||
| + | </p><p>3 clones of each plate have been picked and inoculated in 5 ml LB/cam o/n. | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=150" title="Edit section: Other Work">edit</a>]</span> <span class="mw-headline" id="Other_Work_10">Other Work</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=151" title="Edit section: sequencing of clones">edit</a>]</span> <span class="mw-headline" id="sequencing_of_clones_2">sequencing of clones</span></h4> | ||
| + | |||
| + | <p>Sequence of Blue light promotor with downstream RBS+lacZ is correct. | ||
| + | </p><p>Sequence of first 1000 bp of PCR-Product shows one possible silent mutation in PcyA (Phycocyanobilin:ferredoxin oxidoreductase) gen on Position 45 T->C. Resulting triplet is CCC instead of CCT, which both code for proline. | ||
| + | </p><p>Rest of PCR product should be sequenced aswell? Two more primers would be necessary to cover whole sequence. | ||
| + | </p><p>Or: functionality check using CP919 strain should result in comparable Miller Unit output between PCR product only and red light original part K322127 as CP919 is Φ(OmpC-lacZ)10-25 | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=152" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_14">Testing</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=153" title="Edit section: Miller Assay with blue light construct">edit</a>]</span> <span class="mw-headline" id="Miller_Assay_with_blue_light_construct_2">Miller Assay with blue light construct</span></h4> | ||
| + | |||
| + | <p>two cultures in <b>LB medium</b>: | ||
| + | </p><p><b>blue light - lacZ on pSB1A2 in DH5alpha</b> with three incubation conditions: <b>lighted in room light, dark and blue light</b> | ||
| + | </p><p>cpH8 in DH5 alpha as a control under room light | ||
| + | </p><p>conditions of bacterial growth: 23,5 °C, shaken, both cultures <b>grown in the dark</b> prior to the experiment, two hours before the experiments: cultures were subdivided and lit as described | ||
| + | </p><p>volume of bacterial suspension used: <b>50 µl (or dilutions with LB medium)</b> | ||
| + | </p><p>incubation time for Miller Assay: 5 min (37 °C) | ||
| + | |||
| + | </p><p><br /> | ||
| + | Start of Assay: | ||
| + | - measurement of Abs(600nm) | ||
| + | </p><p>- new eppi with 0,5 ml of Zbuffer + 20 μl of freshly prepared 0,1% SDS+ 40 μl of Chloroform (under fume hood) in 2 ml tube | ||
| + | </p><p>- 50 μl (as a dilution 25 µl + 25 µl LB medium or 10 μl + 40 μl LB medium) of the samples are taken and transferred to the Zbuffer | ||
| + | </p><p>- mix the solution by vortexing for 10 s (all samples with equal vortexing time) | ||
| + | </p><p>- transferring of 100 μl supernatant to 96 well plate (for photometer) | ||
| + | </p><p>- initiation of assay with 20 μl of ONPG (4mg/ml) = START | ||
| + | </p><p>- incubation at 37 °C | ||
| + | </p><p>- stop reaction with 50 μl of 1 M Na2CO3 = STOP after exactely 5 min | ||
| + | </p><p>- measurement of Abs (420nm) and Abs (550nm) | ||
| + | </p><p>- <b>when solution was too yellow (OD > 2.0), stopped mixtures were diluted 1 : 7 in dd H2O</b> | ||
| + | </p> | ||
| + | |||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=154" title="Edit section: Results">edit</a>]</span> <span class="mw-headline" id="Results_14">Results</span></h3> | ||
| + | <p><a href="/wiki/index.php?title=File:Miller_Assay_tabelle_12.9.2011.jpg" class="image"><img alt="Miller Assay tabelle 12.9.2011.jpg" src="/wiki/images/2/25/Miller_Assay_tabelle_12.9.2011.jpg" width="503" height="1651" /></a> | ||
| + | </p><p><br /> | ||
| + | <a href="/wiki/index.php?title=File:Miller_Assay_Diagramm_12.9.2011.jpg" class="image"><img alt="Miller Assay Diagramm 12.9.2011.jpg" src="/wiki/images/6/67/Miller_Assay_Diagramm_12.9.2011.jpg" width="853" height="508" /></a> | ||
| + | </p><p>Problems: | ||
| + | 1. did cells grow up to 20 h and act normally? | ||
| + | </p> | ||
| + | <h2><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=155" title="Edit section: 13-09-2011">edit</a>]</span> <span class="mw-headline" id="13-09-2011">13-09-2011</span></h2> | ||
| + | |||
| + | <p><b>People: Simon, Anna, Alex, Thorsten</b> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=156" title="Edit section: Other Work">edit</a>]</span> <span class="mw-headline" id="Other_Work_11">Other Work</span></h3> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=157" title="Edit section: Electrocompetent cells">edit</a>]</span> <span class="mw-headline" id="Electrocompetent_cells_2">Electrocompetent cells</span></h4> | ||
| + | <dl><dd>new electrocompetent cells (DH5alpha) were made by the following protocol: | ||
| + | </dd></dl> | ||
| + | |||
| + | <dl><dd><dl><dd>20 ml LB medium was inoculated with 1 µl electrocompetent DH5alpha (kindly provided by Andrea Mückl) | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>350 ml LB medium were inoculated with 15 ml of the overnight culture and incubated at 37°C until a OD(600) of 0,7 was reached | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>the medium was distributed on falcons and the cells were centrifuged at 4500 rpm for 20 min at 4°C | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>after discarding the supernatant 350 ml of sterile ice-cold 10% glycerol was added and the pellets were resuspended using a pipet | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>the resuspension was centrifuged at 4500 rpm at 4°C for 11 minutes | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>after discarding the supernatant the cells were resuspended again in 350 ml of ice-cold 10% glycerol (cells kept chilled all the time) | ||
| + | </dd></dl> | ||
| + | |||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>and again centrifuged at 4500 rpm at 4°C for 11 minutes | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>the supernatant was discarded and the cells were resuspended in the remaining supernatant | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>the cells were dispensed in 40 µl aliquots and stored at -80°C for further use | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <p><br /> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=158" title="Edit section: Cloning">edit</a>]</span> <span class="mw-headline" id="Cloning_20">Cloning</span></h3> | ||
| + | |||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=159" title="Edit section: AND-Gate">edit</a>]</span> <span class="mw-headline" id="AND-Gate">AND-Gate</span></h4> | ||
| + | <p>Minipreps of picked cones on 12-09-11 were done according to Metabion's instructions. Concentrations: | ||
| + | </p><p>2a: c = 34.5 ng/µl | ||
| + | </p><p>2b: c = 68.0 ng/µl | ||
| + | </p><p>3a: c = 118 ng/µl | ||
| + | </p><p>3b: c = 65.0 ng/µl | ||
| + | </p><p>5a: c = 122 ng/µl | ||
| + | </p><p>5b: c = 71.5 ng/µl | ||
| + | </p><p>6a: c = 122 ng/µl | ||
| + | </p><p>6b: c = 119 ng/µl | ||
| + | </p><p>6c: c = 51.0 ng/µl | ||
| + | </p><p>After restriction digest using E and P, samples were separated on a 0.8 % gel: | ||
| + | |||
| + | </p><p><a href="/wiki/index.php?title=File:110913_analytischer_verdau_and-gate_Thorsten.jpg" class="image"><img alt="110913 analytischer verdau and-gate Thorsten.jpg" src="/wiki/images/5/51/110913_analytischer_verdau_and-gate_Thorsten.jpg" width="1077" height="996" /></a> | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=160" title="Edit section: Vector prep pSB1A3 and pSB1C3">edit</a>]</span> <span class="mw-headline" id="Vector_prep_pSB1A3_and_pSB1C3">Vector prep pSB1A3 and pSB1C3</span></h4> | ||
| + | <dl><dd>The restricted vectors ID10 pSB1A3 and ID12 pSB1C3 from 30-08-2011 were applied onto a 1 % agarose gel and prepped using the Wizard SV gel purification kit. | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>The vector pSB1A3 was single cut, while pSB1C3 was cut with E and P (from K322127). The expected lengths are about 2 kbp for pSB1C3 and about 3 kbp for pSB1A3, as it also contains a RFP cassette. | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | <dl><dd><dl><dd>The measured concentrations were 12.5 ng/µl for pSB1A3 and 11.5 ng/µl for pSB1C3, in a resuspended volume of 50 µl. | ||
| + | </dd></dl> | ||
| + | </dd></dl> | ||
| + | |||
| + | <p><a href="/wiki/index.php?title=File:110913_Verdau_pSB1A2_und_pSB1C3_Thorsten_nach_Schnitt_bearbeitet.jpg" class="image"><img alt="110913 Verdau pSB1A2 und pSB1C3 Thorsten nach Schnitt bearbeitet.jpg" src="/wiki/images/6/6a/110913_Verdau_pSB1A2_und_pSB1C3_Thorsten_nach_Schnitt_bearbeitet.jpg" width="844" height="755" /></a> | ||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=161" title="Edit section: Testing">edit</a>]</span> <span class="mw-headline" id="Testing_15">Testing</span></h3> | ||
| + | <p><b>no Miller Assay today</b> | ||
| + | </p> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=162" title="Edit section: Inoculation of o/n-cultures for tomorrow´s Miller Assay">edit</a>]</span> <span class="mw-headline" id="Inoculation_of_o.2Fn-cultures_for_tomorrow.C2.B4s_Miller_Assay_2">Inoculation of o/n-cultures for tomorrow´s Miller Assay</span></h4> | ||
| + | |||
| + | <p>10 ml LB+antibiotic in 50 ml-falcon tube | ||
| + | </p><p>add 20 µl (= 1:1000 dilution) of yesterday´s o/n-cultures: | ||
| + | </p><p>in LB medium: | ||
| + | </p> | ||
| + | <ul><li>bluelight + lacZ in DH5a (Amp) | ||
| + | </li><li>neg. contr. Cph8 in DH5a (Kan) | ||
| + | </li></ul> | ||
| + | <p><br /> | ||
| + | in M9 medium: | ||
| + | </p> | ||
| + | <ul><li>redlight + lacZ (trunc.) in DH5a (Cam) | ||
| + | </li><li>neg. contr. Cph8 in DH5a (Kan) | ||
| + | </li></ul> | ||
| + | <p><br /> | ||
| + | <b>Wrap falcons with aluminium foil, so growth can occur in the dark!</b> | ||
| + | </p><p>Shake o/n @ 37°C, 180 rpm | ||
| + | |||
| + | </p> | ||
| + | <h3><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=163" title="Edit section: Other Work">edit</a>]</span> <span class="mw-headline" id="Other_Work_12">Other Work</span></h3> | ||
| + | <p>1. preparation of Zbuffer and Na2CO3 solution for next day | ||
| + | </p><p>2. Transformations: | ||
| + | </p> | ||
| + | <dl><dd> chemical transformation of pSB1AK8 T7prom RBS lacZ (plasmid from clone 2c, 09.09.2011) in BL21 DE3. The BL21 cells we received from Lars Mitschke turned out to be chemically competent cells. Transformation was performed based on a protocol from openwetware.org. For detailed description of the protocoll see Methods. | ||
| + | </dd></dl> | ||
| + | <dl><dd> electroporation of Part R2 (= RBS + lacZ) in pSB1A2 using today's and the old electrocompetent DH5alpha cells. | ||
| + | </dd></dl> | ||
| + | <h4><span class="editsection">[<a href="/wiki/index.php?title=Labbook_Part_3&action=edit&section=164" title="Edit section: Sequencing results">edit</a>]</span> <span class="mw-headline" id="Sequencing_results">Sequencing results</span></h4> | ||
| + | |||
| + | <p>pSB1AK8_T7prom_RBS_lacZ_VF2_110909_clone_2c-GATC-VF2-545457: Sequence shows one missing T in scar between T7promoter and RBS of lacZ. Rest of sequence is fine. | ||
| + | </p><p>Sequence | ||
</p> | </p> | ||
</div> | </div> | ||
Revision as of 17:17, 15 September 2011
[edit] Light sensor systems and AND-Gate cloning Part III Alex/Simon/Bea/Thorsten
[edit] 24-08-2011
People: Alex, Simon, Bea, Thorsten
[edit] Cloning
[edit] Ligation
Three ligations in total were conducted. K238013 (S,P) was quick-ligated with lacZ (X,P), rbs (S,P) with T7Pol (X,P) and T7Prom (S,P) with lacZ (X,P).
[edit] Transformation
The ligations were transformed into DH5alpha. Additionally, K238013 (from23-08-2011) was transformed into DH5alpha. The ligation of K238013 with lacZ is plated onto Amp and Cm, rbs with T7pol is plated onto Amp, T7Prom with lacZ onto Kan and K238013 is plated onto Amp and Cm.
[edit] Testing
The two cultures of K322127 in DH5alpha incubated over night with red light (ca. 630 nm) were split. One half was further incubated with red light, while the other half was incubated with sun light and light from a table-lamp over night.
Test of negative controls on S-Gal: T7prom (Kan) transformed into DH5alpha and K322127 (Cm) transformed into BL21 were inoculated onto S-Gal plates with the respective antibiotics. The are incubated over night, and should not yield black colonies, as they both should not be able to express functional lacZ.
[edit] Results
Expression test of reporter plasmid: The cultures with ligation of T7prom and lacZ transformed into BL21 and induced with IPTG showed black colonies on the S-Gal plates. However, the cultures, which were NOT induced also showed black colonies of the same intensity. At the current point, we cannot sufficiently explain this.
[edit] Red light sensor assembly (Bea and Thorsten)
aim:
Ligation of red light sensor didn't work, yet. Therefore we ligated PCR product of BBa_K322127(X and P cut) into pSB3C5(S and P cut) to verify correct sites. Subsequent amplification and recutting of this part should lead to correct ligation with SupD.
In parallel, PCR was performed again in case of failed ligations with existent PCR-products.
Materials:
digest:
pSB3C5: S and P cut by Alex on 23.08.11, CAM resistance, 2,7 kbp vector backbone
PCR product of BBa_K322127 with primers BBa_K568000, derived from Biomers, sequence see below, 3,8 kbp (Florian 6.7.11 labelled with "K322127 PCR product aus gel ausgeschnitten")
pcr:
Template: BBa_K322127 ("Edinburgh part") conc: 150 ng/µl
primers (synthesized by biomers, 29.6.11, stock: 100µM):
- BBa_K568000_fwd: tatatctatatcgaattcgcggccgcttctagagtttacggctagctcagtcctaggta (59bp)
- BBa_K568000_rev: cgtgccggcggctgcagcggccgctactagtaagtccattctccccaaaaatg (53bp)
Procedure
digest:
10 µl PCR Product was cut with 1 µl XbaI and 1 µl PstI in 5 µl NEB4-buffer (total volume 50 µl) for 2h at 37°C with subsequent heat inactivation at 80°C for 20 minutes. Cut PCR-product was loaded on a 1% Agarose Gel in TAE buffer and run at 120 V for 1 h.
PCR:
amplification with Taq PCR Kit (NEB) according to Florian at 5.7.11. No water control was run due to lacking remaining buffer volume.
- 5 µl 10x standard buffer
- 1 µl dNTP's
- 0,25 µl Tag DNA polymerase
- 0,5 µl primers, respectively
- 0,4 µl Template DNA
- add up to 50 µl with sterile water
PCR-program:
- see 5.7.11
Bands were cut from Gel and DNA was isolated using Squeeze N Freeze (protocol see Methods)
[edit] Results
1% Agarose Gel of new PCR-product (line 3) and cut PCR-product (line2) with 2 log ladder (line 1)
cut PCR Fragment with correct size (3,8 kbp) was frozen after isolation. PCR yielded some short products with variable length. Used PCR-Kit is not suitable for amplification of long fragments. Experiment will be repeated tomorrow with correct Kit.
[edit] 25-08-2011
People: Alex, Simon, Bea, Thorsten
[edit] Cloning
[edit] Digestion
R2 (red writing: MiniPrep iGEM R2 31.5.11) was digested with X,P and T7prom (black writing: 17.6.11 T7) with S,P. After heat-inactivation, GLP was added to the digested DNA. The samples were applied onto a 1 % Agarose gel. The samples, along with those from the PCR (see below), were applied as follows: 2lod DNA ladder (5 ul); R2 (20 ul); R2 (20 ul); T7prom (20 ul); T7 prom (20 ul); PCR cut; positive control; PCR approach 1; PCR approach 1; PCR approach 2; PCR approach 2; PCR approaches 1 and 2 pooled. The gel was run for 1:30 h at 110 V. The following bands were expected: 3.1 kbp for lacZ with rbs and 2 kbp for vector pSB1A2. 3.4 kbp for T7prom in pSB1AK8. The bands are excised from the gel and the DNA is recovered using freeze 'n squeeze.
The original file before cutting of the bands seems to be corrupt.
[edit] Ligation
In the next step, K238013 (from 23-08-2011; cut with S,P) was ligated with rbs&lacZ (=R2; X,P), rbs (from 22-08-2011; S,P) with T7pol (from 23-08-2011; X,P) and T7prom (S,P) with rbs&lacZ (X,P). Each time, ligation was performed as normal and as quick-ligation. After that, the transformed cells were plated onto LB (Amp) plates and incubated over night.
[edit] New PCR of K322128 using Phusion High-Fidelity PCR-Kit (NEB/Finnzymes)
Material
BBa_K322127 with primers BBa_K568000, derived from Biomers, 3,8 kbp cut vector pSB1A2 digestion with X,P from Alex on 25.8.2011 (Florian 6.7.11 labelled with "K322127 PCR product aus gel ausgeschnitten")
Procedure
using same protocol as Florian on 11.7
PCR-Program:
1) Initial Denaturation: 98°C, 4'
2) 30 cycles of:
Denat.: 98°C, 30 s Annealing: 60°C, 15 s Extension: 72°C, 2´ 30 s
3) Final extension: 72°C, 8'
4) Hold: 4°C, oo
samples
only 2 samples were prepared using each:
35,5µl of ddH2O 10 µl5x buffer 1 µl dNTPS 1 µl of template DNA K322127 (1,5ng/µl ) 1 µl of Primer fwd (10µM) 1 µl of Primer rev (10µM)
only the 10kB positive control was done:
34µl of ddH2O 10 µl5x buffer 1 µl dNTPS 1 µl of template DNA (template for positive control) 2,5 µl of Primer (10kB-Mix)
after pcr the samples were applied on a 1% Agarose-gel
The bands are excised from the gel and the DNA is recovered using freeze 'n squeeze.
two times 10µl of the PCR product were digested with X,P
the digestion and the pcr product were stored at -20°C
[edit] Ethanol precipitation of digested PCR product
Material: PCR product cut with X,P from the 24.8.2011 protocol see Methods the sample was applied on an agarose gel because the nanodrop indicated no DNA
[edit] Result
The gel showed that no DNA was yielded (see picture Restriction & Ligation)
[edit] Repeated Digest and Ligation of PCR Product
Material: PCR product of BBa_K322127 with primers BBa_K568000, derived from Biomers, 3,8 kbp cut vector pSB1A2 digestion with X,P from Alex on 25.8.2011 (Florian 6.7.11 labelled with "K322127 PCR product aus gel ausgeschnitten") Procedure: The PCR product was digested with X,P. After heat-inactivation, GLP was added to the digested DNA. The samples were applied onto a 0,8 % Agarose gel. The gel was run for 1:30 h at 110 V. The bands are excised from the gel and the DNA is recovered using freeze 'n squeeze. Ligation of the cut fragment with the vector pSB1A2 (cut with X,P; see Restriction & Ligation above) using quick ligation protocol see Methods
[edit] Result
[edit] Testing
[edit] SDS-PAGE
1 OD_600 each from the K322127 in DH5alpha cultures from yesterday was taken. The OD_600 were: 0.835 for the first colony exposed to bright lamp (bright 1); 0.84 for the second colony exposed to bright lamp (bright 2); 1.685 for the first colony incubated with red light (red 1); 1.855 for the second colony incubated with red light (red 2). The cells were centrifuged at 13000 rcf for 8 min. After resuspension in 100 ul loading buffer, the tubes were incubated for 10 min at 95 °C. After that, the samples (5 ul and 10 ul) were loaded onto a 10 % SDS-PAGE with stacking gel. Pageruler was used as protein ladder. The gel was run for 2:00 h at 80 V, which was increased to 120 V when the buffer front reaching the separation gel. After that, the gel was stained with Coomassie blue and subsequently destained.
The gel does not show elevated intensities of the band at around 10 kDa for lacZ alpha peptide, which would have been expected.
[edit] T7 promoter in vitro assay
As proposed yesterday, a second in vitro assay was performed to find out if the plasmids contain a T7 promoter. This time, a third sample was made for each plasmid (same plasmids as yesterday, see above). In this sample (labeled "#xi") the RNase that we assume to be present were inhibited using RNA secure prior to addition of T7 polymerase and incubation. See methods for detailed procedure. The 2% agarose gel was run at 110 V, 400 mA for 1:30 hours.
[edit] Other Work
[edit] K322127 Inoculation & Incubation:
The following inoculations were performed:
- "K238013 in DH5 alpha", plate date: 24.08.2011, yesterday's transformation, in LB Amp
- "BM28 from Jeanette Winter", plate date: 31.05.2011, in LB Amp, Kan
- "green light 2", plate date: 27.6.2011, in LB Cm
- "green light 3", plate date: 27.6.2011, in LB Cm
- "green light 4", plate date: 27.6.2011, in LB Cm
- "K322127 in DH5 alpha, Kolonie 1, Licht-induziert", plate date: 22.08.11, in LB cm (the picked colonies were the black ones after over night incubation in light)
- "K322127 in DH5 alpha, Kolonie 2, Rotlicht-deaktiviert", plate date: 22.08.11, in LB cm (the picked colonies were white ones after over night incubation in red light)
Incubation over night at 37 °C, 200 rpm.
[edit] Results
The PCR was successful (see picture Restriction & Ligation), the positive control and both samples yielded DNA that is located at the right spot according to their length positive control (10kB) samples (3,8kB)
[edit] Results
24.08.11
T7 promoter in vitro assay
The samples labeled "#xi" were treated with RNA secure to inhibit potentially present RNase. The gel photo shows blurry signals in all of these lanes which aren't visible in lanes "#x" and "#xa". This proves that RNase is present in the DNA samples used in the assay. Also, the fact that the RNA signals are quite large and strong shows leads us to the conclusion that the examined plasmids all contain a T7 promoter. Sample 1 contains two plasmids (about 6.6 kb and 3.5 kb) which is the reason for the bad sequencing data. Samples 2, 3, 5 and 6 contain one 6.6 kb plasmid each. This should be our correct reporter plasmid, part 2b, containing T7 promoter and lacZ. To verify this, a sample shall be sequenced.
S-Gal negative Control:
DH5alpha cells transformed with T7prom yielded white colonies on the S-Gal plates. However, BL21 cells transformed with K322127 yielded all black colonies. Most likely, our strain of BL21 is unsuited for expression tests using S-Gal (might constitutively express lacZ).
Expression test of K322127
After over night incubation, both plates incubated in red light showed white colonies only. Of the two plates incubated under the lamp, one did not show any colonies. The other one showed black colonies. --> First evidence that part K322127 works as expected!!! To further quantify and characterise this: Miller assay? Other methods?
[edit] 26-08-2011
People: Alex, Simon, Bea, Thorsten
[edit] Other Work
Part-request: Parts requested from the registry for backup if the ligation of the PCR product will fail again:
| Part | Team | Library | Plate | Well | Plasmid | Resistance |
|---|---|---|---|---|---|---|
| BBa_K322123 | iGEM10_Edindburgh | 2010 Submisions Samples 001 | Shipment: 00694 | 4D | pSB1C3 | C |
| BBa_K322124 | iGEM10_Edindburgh | 2010 Submisions Samples 001 | Shipment: 00694 | 4E | pSB1C3 | C |
And Gate plasmid: PCR-Product from Edinburgh
New media and plates: LB, LB for Agar plates (Kan and Amp?) and Luria medium were prepared. Plates were prepared.
Sequencing:
The reporter plasmid clone 5 was handed in for sequencing at GATC-Biotech. The primer used for sequencing is "Primer pSB1A2 und pSB1AK8 fwd".
Skype talk with iGEM Team Freiburg
[edit] Cloning
Miniprep & Glycero-Stock: The cultures inoculated yesterday on LB were frozen at -80 °C as glycero-stocks. The culture of K238013 (pSB1A2) in DH5alpha was prepped. The resulting DNA yielded a concentration of approximately 300 ng/ul.
Repetition of transformations:
The transformations from yesterday (ligation using ligation protocol) were repeated using DH5alpha cells (kindly provided by Andrea). Transformations were done for reporter plasmid (part 2b) colony 5 from DATUM, K238013 with lacZ, rbs with T7pol and T7prom with lacZ. After incubation for one hour, the cells were plated on LB Amp (K238013 with lacZ; rbs with T7pol) and on LB Kan (reporter plasmid; T7prom with lacZ) and incubated over night.
Ligation of new PCR product with pSB1A2
Material:
cut PCR (X,P) product from K322127 from PCR 25.8.2011 cut pSB1A2 (X,P) from Alex from 25.8.
procedure:
we purified PCR product using Zymo DNA Clean +Concentrator 25
ligation:
sample 1: 15µl PCR product 5µl vector sample 2: 10µl PCR product 10µl vector
Transformation of the ligation and R2 as transformation control:
the two samples from purifiying and the part R2( OmpR in pSB1A2) as a positive control were transformed via electroperforation in competent DH5alpha cells that were kindly supplied by Andrea Mückl.
[edit] Testing
Testing K322127
Material: inoculated cultures of DH5alpha with K322127 from 25.8 Procedure: 100 µl of the culture was spread on S-gal plates with Kan and amp in dark Incubation of the plates at room temperature (30°C) under light of diode with maximum of 630nm (turn-off diode) for 2 hours afterwards incubation at light at 37°C for 19 hours afterwards 4°C in the incubator: one culture with aluminium TUM logo put on top one culture in the box under light of turn-off diode (negative control) one culture on top of the box, light from turn-off diode from below, light from the incubator from top only on cut out TUM logo in aluminium foil
[edit] Results
26-08-2011
Transformations: The transformations from yesterday, including K238013 & lacZ, rbs & T7pol, T7prom & lacZ as well as PCR product & Vector, did not work at all. There were no colonies on the plates.
Sequencing: More data from the sequencing from 19-08-2011 arrived. The newly ligated K238013 & double terminator from 17-08-2011 does contain the double terminator. However, as witnessed before, the blue light promotor K238013, which was part of the plasmid prior to ligation, is missing. We are still awaiting the data from another sequencing from the same construct, but from another ligation.
Planning for next week: We need to prepare new electrocompetent DH5alpha cells. EVERYONE, who has free time to spare, please come to the lab and help. We might also be able to get new BL21 (DE3) cells from Prof. Buchner. If anyone wants to get them, contact Lars Mitschke at the Lehrstuhl of Prof. Buchner and talk to him about the request, which Alex asked about. BL21 (DE3) contain a T7polymerase, which can be induced by IPTG. This would be great use in order to test our reporter plasmid, i case the ligation worked (which we should know on Monday).
[edit] 29-08-2011
[edit] Cloning
inoculation the following were inoculated: T7 Promotor with lacZ 2x K322127 in DH5alpha
[edit] Retransformation of reporter plasmid clone 5
the reporter plasmid clone 5 (118ng/µl) was diluted to 50 pg/µl and to 20 ng/µl
1 µl of each dilution was transformed into DH5alpha
[edit] Transformation of RBS
0,42 µl of RBS (47,5 ng/µl) were transformed into DH5alpha
[edit] Results
26-08-2011
transformations
were only successful for R2, T7 Promotor with lacZ, reporter plasmid colony 5
testing of K322127
only very small colonies on the plate with no coloring plate that was illuminated with turn-off light and light from the incubator dried out. no black colonies could be detected. Incubation of plate with TUM pattern was continued. Plate was irradated with a light bulb.
sequencing of reporter plasmid colony 5
Bases in squared brackets are optional and belonging to another sequence. Due tu overlapping base shifts exact assignment of bases is difficult
Sequencing reveals mixed clones. Correct Sequence with T7 promotor - RBS – lacZ is probably present beneath construct with T7 promotor lacking. Preparation of RBS-LacZ vector was contaminated with uncut or religated vector. Since plasmid length was ok, construct should be present.
next steps:
Retransformation with 50 pg and 20 ng MiniPrep DNA will be performed with subsequent sequencing of 4 clones. In parallel new construct from Alex will be sequenced.
[edit] 30-08-2011
People: Thorsten, Bea
[edit] Results
29.8.11
Re-Transformation of part 2b clone 5 with 20 ng vector was succesful. 50 pg vector didnt yield any colonies. RBS was tranformed succesfully. 5 ml LB o/n cultures with apropriate antibiotica were inoculated and incubated at 37° C shaking. Mini Prep and sequencing will be performed at 31.8.11
[edit] Cloning
[edit] Electrocompetent cells
- new electrocompetent cells (DH5alpha) were made by the following protocol:
- a 20 ml Luria medium overnight culture was inoculated with electrocompetent DH5alpha (kindly provided Andrea Mückl) yesterday
- 350 ml Luria medium were inoculated with 15 ml of the overnight culture and incubated at 37°C until a OD(600) of 0,7 was reached
- the medium was distributed on falcons and the cells were centrifuged at 4500 rpm for 20 min at 4°C
- after discarding the supernatant 350 ml of sterile ice-cold 10% glycerol was added and the pellets were resuspended using a pipet
- the resuspension was centrifuged at 4500 rpm at 4°C for 11 minutes
- after discarding the supernatant the cells were resuspended again in 350 ml of ice-cold 10% glycerol ( cells kept chilled all the time)
- and again centrifuged at 4500 rpm at 4°C for 11 minutes
- the supernatant was discarded and the cells were resuspended in the remaining supernatant
- the cells were dispensed in 40 µl aliquots and stored at -80°C for further use
[edit] Planning
In order to get ligations working, purification steps will be performed using Promegas Wizard PCR and Gel clean up Kit. We are going to ligate PCR product into pSB1C3 and upstream of SupD-tRNA. In parallel, T7ptag will be ligated into AND-Gate Plasmid, which will be pSB1C3. [First step of assembly]
Then, synthesis product, which contains parts from SupDtRNA to RBS J44001, will be ligated into succesful AND-Gate Plasmid-T7ptag. The last step will be ligation of PCR-product into this construct.
Excel sheets can be found in DropBox folder "protocols".
planned parts
PCR product ligated into pSB1C3 will be a new part "red light sensor without lacZ".
PCR product ligated with SupD will be a new part as well.
Synthesis product is another maybe not so useful part which will be send into the registry.
Reporter plasmid will be another Part. This one was called "part 2B" in recent cloning steps and needs further validation (see 29.8. retransformation and sequencing)
[edit] Restriction digest
All digestions were complete and running on the expected lengths.
[edit] Purification of restricion digests from gel with Wizard Kit
the following items were purified using the Wizard Kit according to manufacturer's instructions and the concentration was determined by nanodrop:
1: 24,5 ng/µl
2: 24,5ng/µl
3: 24,5 ng/µl
5: 20,5 ng/µl
9: 20,0 ng/µl
10: 23 ng/µl
11: 21,5ng/µl
12: 29,5 ng/µl
13: 20,0 ng/µl
for more details see restriction digest table
[edit] Inoculation
the following overnight cultures were inoculated:
RBS reporter plasmid clone 1-5 K322127 from plate from 24.8.2011
[edit] 31-08-2011
[edit] Results
30.8.11
Electrocompetent cells show good transformation efficiency. 100µl 10µl and 1µl of test transformation of 30 ng BBa_K322127 were plated on LB/cam plates. 1 µl yielded ca 400 colonies which refers to a tranformationefficiency of 1.3*10^7 cfu/µg BBa_K322127 in pSB1C3. 1*10^8 cfu/µg pUC19 is expected, so 1.3*10^7 cfu/µg vector with insert seems to be ok.
cph8 from Uppsala was succesfully transformed into DH5alpha. Cells have been 1:3 diluted and 20 ng plasmid was used. Output was ca 10^6 - 10^7 cfu/µg. Plate will be stored at 4°C til inoculation of o/n culture with subsequent minipreparation.
[edit] Cloning
[edit] Ligation
[edit] Minipreparation of DNA
The following concentrations were gained through minipreparation:
RBS : 86,5 ng/µl
reporter plasmid clone 1: 135 ng/µl
reporter plasmid clone 2: 234 ng/µl
reporter plasmid clone 3: 102 ng/µl
reporter plasmid clone 4: 119 ng/µl
reporter plasmid clone 5: 96 ng/µl
The reporter plasmid clone 1-5 were sent to be sequenced at GATC.
[edit] Testing
the overnight culture of K322127 was used to inoculate a 100 ml culture in LB which was incubated for 5 hours at 37°C
at an OD of 1.6 50 ml of the cells were centrifuged at 4500 rpm the supernatant discarded
the cells were resuspended in remaining 1 ml of LB
100 ml of LB media with s-gal was prepared and autoclaved according to protocol see Methods
the resuspended cells were added to the medium when it reached 40°C
the media was given in thee plates
two plates were incubated at 37°C under turn-off light (diode max. 630 nm)
the TUM cookie cutters were inserted in one plate that was then incubated under the light of a lamp
[edit] Other Work
new chloramphenicol plates were made
[edit] 01-09-2011
[edit] Cloning
[edit] gel purification of 30.8.11 digest
20 µl of remaining digestions (made on 30.8.11) were loaded on 1 % agarose gel with 1xTAE. Items 1,2,3,4,5,6,7,8,9,10,12 were used. This samples were cut using 350nm UV illumination as short as possible. Cut gel slices were subsequently purified using Promegas Wizard Kit according to manufacturers instructions.
Determination of concentration with Nanodrop resultet in not reliable data. Therefore quantitative 0.7 % agarose gel was run with 5 µl of each sample. 5 µl 2 log ladder was used (100 µg/ml). PCR Band (3.8 kbp) in line 2 was estimated to 75 ng. This gives aproximately 15 ng/µl. Intensities of 2 kbp band in line 4 as well as lines 5, 6, 7, 10 and 11 correspond to approx. 150 ng amount (--> 30 ng/µl).
Due to fly-by-night intensity of ladder those concentrations will be determined again on 02.09.11 for further ligations. Ligations today will be done with the following concentrations
[edit] o/n ligation with T4 Ligase
P, S opened Vector containing blue light promotor was ligated with RBS-LacZ insert. Due to huge insert lengths, insert:vector ratios were choosen as can be seen in ligation table.
Ligation was conducted at 16° C in thermocycler o/n using 20 µl sample volume and 1 µl T4 DNA ligase. Examplary calculation of I/V ratio for 6x molar excess of insert (source: http://openwetware.org/wiki/DNA_Ligation):
[edit] Testing
inoculation of two cultures with 5 µl of the glycerol stock of K322127 in DH5alpha
the cultures were incubated at turn-off light (diode max. at 630 nm) at 37°C
new transformation of K322127 (150ng/µl) 0,5 µl were used
[edit] Testing
[edit] Preparing lacZ-ONPG or also called Miller Assay
[edit] Other Work
[edit] Part design in the registry
edit the general information of the part : http://partsregistry.org/wiki/index.php?title=Part:BBa_K568000&action=edit
[edit] others
the primer for the sequencing of reporter plasmid clone 1-5 from yesterday is sent to GATC
Lars Mitschke kindly provided the E. coli strain BL21 (DE3), this strain contains a T7 Polymerase which can be induced with IPTG.
[edit] Results
31-08-2011
transformations
no colonies on the plates for all ligations
testing of K322127
the plate that was incubated under light showed no coloring but the plate became intransparent which indicates that the bacteria grew
plates incubated under turn-off light (diode max. 630 nm) became intransparent as well; this plates were used for further testing ( see testing of K322127 )
[edit] 02-09-2011
[edit] Cloning
[edit] Digest of T7 promoter
Full volume of digest was loaded onto gel (50 µl digest + 10 µl 6 x Gel loading buffer)
Band with correct size of 3,5 kbp was cut using 365 nm illumination. Illumination time was kept as short as possible, picture was taken after cutting gel slice.
[edit] Gel purification of T7 promotor
Band was purified using Promega's Wizard Gel purification Kit according to manufacturer's instructions. Eluted DNA was subsequently quantified with an analytical 0.7 % agarose gel (120 V for 45 min)
Band in line 2 corresponds to 120 ng band of marker. Therefore concentration of purified T7 promoter is approx. 24 ng/µl. RBS_lacZ band is approx. 20 ng/µl and pSB1C3 band approx. 16 ng/µl
[edit] Ligation of Reporter Plasmid
digested T7 promoter was ligated with RBS_lacZ from 1.9.11 purification.
Ligation was conducted o/n at 16°C.
[edit] Other Work
[edit] Agar Plates
500 ml of media for ampcillin plates
250 ml of media for s-gal plates were prepared
[edit] Cloning
[edit] Transformation
the ligations from 01-09-2011 (1-7) were transformed into DH5alpha as a transformation control 0,5 of R2 (233ng/) was transformed into DH5alpha
[edit] Testing
[edit] preparing ONPG assay
ONPG solution (4mg/ml)
1 M Na2CO3 were prepared
[edit] Overnight Cultures
new overnight cultures of the new transformation of K322127 (see 01-09-2011) were inoculated
[edit] Results
01-09-2011
Testing K322127
no coloring on plates
[edit] 03-09-2011
People: Bea, Thorsten, Susan, Wolfgang
[edit] Other work
Cloramphenicol stock was produced: 30 mg/ml final concentration in 100% ethanol. 153 mg + 5,1 ml 100% EtOH. 500 µl aliquotes were stored at -20°C.
[edit] Cloning
[edit] Precipitation of Ligations and Transformation
Todays Transformation were done after purifing ligation mix with Glycogen/Ethanol precipitation in order to increase transformed amount and purity of ligated vectors.
All Ligations of 02-09-11 and following items of 01-09-11 ligation were used:
- 1 (pos control pSB1A3single cut (10))
- 2 (PCR(1)-pSB1C3 linearized (3))
- 4 (T7ptag(1)-pSB1C3 (12))
- 6 (blue light(8) - lacZ(9))
Glycogen/ethanol precipitation:
- -20 µl of the ligation mixed with 2 µl NaAc (3M), 0.5 µl glycogen and 22.5 µl isopropanol
- -incubation step for 1 h @ -20 °C
- -15 mins @ 10.000 rpms
- -withdraw supernatant
- -wash pelett with 70 % EtOH ~ 10 µl
- -dry pelett and resuspense in 10 µl water
Transformation via electroporation was done with the whole cleaned 10 µl in 40 µl DH5alpha cells.
[edit] Ligations of PCR product into pSB1C3 and pSB1A3
Following ligations were examined using an excess of vector and 1 µl PCR-product (corresponds to approx 14 ng/µl).
Items 1+2 were incubated at room temperature and items 3+4 were incubated at 37° C for 1h each. 5 µl of ligations were subsequently electroporated into 40 µl undiluted DH5alpha cells.
[edit] Results
02-09-2011
[edit] Testing
no coloring on plates
[edit] Cloning
Single cut vector control(1) showed approx. 50 colonies. ALl ligations plated on Cam plates (2-5) showed no colonies.
Blue light - RBS_lacZ ligations (6+7) showed 5 colonies overall. Clones were inoculated into 5 ml LB/Amp and incubated at 37°C o/n.
[edit] 04-09-2011
People: Bea, Thorsten, Flo
[edit] Results
03-09-2011
[edit] Transformation of purified ligations (02-09-2011)
positive control showed several thousand red colonies. All ligations showed bacterial lawn, including vector control.
Vector prep contains uncut or single cut vector. This result is consistent with sequencing results, which indicate mixed clones. Next steps: Preperation of 46 bp T7 promoter insert with 3 % preparative agarose gel and ligation upstream of RBS_lacZ into opened RBS_lacZ vector. In parallel vector prep of T7 promoter will be repeated on 1% agarose gel with a long gel and run. Uncut and single cut vector will be loaded on gel aswell.
[edit] Transformation of 03-09-2011 ligations
showed following output:
pSB1A3 colonies were possibly red. --> incubated again o/n
http://partsregistry.org/Part:BBa_J04450 --> red color indicates religated or uncut vector clone
4 clones of each successful transformation were picked and inoculated into 5 ml LB medium containing the appropriate antibiotica.
Conclusion:
Further incubations should be done at RT for 1h or o/n at 16°C with subsequent gylkogen/ethanol purification and transformation of whole 10 µl resuspended pellet into 40 µl DH5alpha
[edit] Cloning
[edit] Reporter Plasmid
As recent transformation of reporter plasmid yielded high vector background new preps will be done according to following scheme.
[edit] digest
long 1% preperative agarose gel and 3% preperative agarose gel have been produced and stored in a plastic bag with some 1x TAE buffer at 4°C o/n. Purification will be done tomorrow.
[edit] Minipreps of picked clones of Bluelight_lacZ 01-09-11 ligation (items 6+7)
[Flo]
[edit] Miniprep of 5 o/n cultures [names below protocol] (Metabion Kit)
All centrifugation steps were performed at 13.000 rpm and RT
1. Pellet 2 x 1,5 ml of bacterial suspension in one 2 ml-Eppi, centrifuge for 1 min each
2. Add 250 ul Bact.resuspension buffer, vortex
3. Add 250 ul Cell lysis buffer, invert 5x, incub. approx. 4 min
4. Add 350 ul DNA binding buffer, invert 5x, centrifuge for 10 min
5. Transfer clear lysate on column, centrifuge for 1 min, discard supernatant
6. Add 600 ul Column Wash Buffer, centrifuge for 1 min
7. Transfer column to new 1,5 ml-Eppi
8. Add 50 ul nuclease-free water (from Promega Wizard Kit), incub. approx. 2 min, centrifuge for 1 min
9. Store at -20°C
names:
-Blue Prom + lacZ #1 (= former "blue lacZ Trafo 7, Klon 2")
-Blue Prom + lacZ #2 (= former "blue lacZ Trafo 7, Klon 1")
-Blue Prom + lacZ #3 (= former "blue lacZ Trafo 6, Klon 1")
-Cph8 in DH5a
-K322127
[edit] Preparing glycerol stocks from cultures used above
Prepare 50% glycerol/LB solution by mixing 2 ml 100% glycerol and 2 ml LB
Add 300 ul of 50% glycerol/LB solution to 700 ul bacterial suspension to obtain an end concentration of 15% glycerol
Store at -80°C
[edit] Restriction digest of 5 Minipreps from today
Each plasmid has been cut with EcoRI HF AND EcoRI HF + PstI HF (= 2 digests per plasmid) as follows:
-5 ul Plasmid DNA
-2 ul 10x NEB buffer 4
-0.5 ul EcoRI HF
-(0.5 ul PstI HF)
-12 (11.5) ul ddH2O
end volume is 20 ul
mix, spin down, incub. at 37°C for 1 hour, inactivate at 80°C for 20 min
Add 4 ul 6x loading dye
Store at -20°C until tomorrow
--> quantification on gel 05-09-11
[edit] Testing
[edit] ONPG-Test - Miller Assay
The assay was conducted following the protocol:
3 samples of K322127 in DH5alpha were assayed: 1 sample was incubated for 25 hours in the dark at room temperature 1 sample was incubated for 25 hours in the light at room temperature 1 sample was incubated for 25 hours in the turn-off light at room temperature
- - inoculation of 4 x 2ml LB with 10 μl of overnight cultures of K322127 (see 02-09-2011)
- - light induction when the cultures reach OD of around
- - control with only LB
start of Assay:
- - measurement of Abs(600nm)
- - new eppi with 1 ml of Zbuffer + 20 μl of freshly prepared 0,1% SDS+ 40 μl of Chloroform (under fume hood)
- - 100 μl of the samples are taken and transferred to the Zbuffer
- - mix the solution by vortexing for 10 s
- - let the chloroform settle to the ground and take 700 μl of supernatant for measurement
- - initiation of assay with 140μl of ONPG (4mg/ml) NOTE START TIME
- (in the paper they use 100 μl supernatant plus 20 μl of ( 4mg/ml but we need more for measurement in cuvette)
- -incubation at room temperature until yellow color develops
- -stop reaction with 350 μl of 1 M Na2CO3 NOTE STOP TIME (we upscaled the paper uses 50 μl)
- - measurement of Abs (420nm) and Abs(550nm)
Abs 420 nm should be less than 1 and more than 0,5
[edit] Result
incubation for 146 min because no visible yellow color developed;
not completely clear how to calculate the miller units:
here miller units are calculated as following:
units= 1000* (Abs 420-1,75 Abs550) /(time(min)*Abs 600/(11,6*1,7)))
[edit] Other Work
[edit] S-Gal plates
Flo did Minipreps of cph8 from Uppsala and K322127. Nanodrop yielded no reliable data. --> quantification on gel
[edit] 05-09-2011
People: Bea, Thorsten, Anna
[edit] Testing
[edit] Miller Assay
5 newly inoculated cultures were assayed:
after 5 hours of incubation the inducation was started and the first assay started:
induction start means: incubation at room temperature of: K322127 in DH5alpha in the light of lamp K322127 in DH5alpha in the dark K322127 in DH5alpha in the turn-off light K322127 in BL21in the light cpH8 in DH5 alpha in the light
a new protocol was used for this assay:
start of Assay:
- measurement of Abs(600nm)
- new eppi with 0,5 ml of Zbuffer + 20 μl of freshly prepared 0,1% SDS+ 40 μl of Chloroform (under fume hood)
- 0,5 ml of the samples are taken and transferred to the Zbuffer
- mix the solution by vortexing for 10 s ( all samples equal vortexing time)
- initiation of assay with 200μl of ONPG (4mg/ml) NOTE START TIME
-incubation at room temperature for see table
-stop reaction with 500 μl of 1 M Na2CO3 NOTE STOP TIME
- measurement of Abs (420nm) and Abs(550nm)
[edit] Cloning
[edit] Analytical gel of Bluelight_lacZ, cph8 from Uppsala and K322127 testdigest
Size of bands look fine. Expected: 3093bp(lacZ)+86bp(Bluelight)= 3179 bp
--> Send clone 2 for sequencing or load RBS_lacZ digest along with ligated construct on gel and check for 86 bp difference.
cph8(2,2kbp) and K322127(4kbp) are running on their correct sizes.
[edit] Miniprep and testdigest of picked colonies on 04-09-2011
Miniprep of yesterdays picked clones were done. Concentration was determined using Nanodrop.
T7 Pol in pSB1C3 (ligation 4 from 1.9.)samples:
clone 1 : 58,5 ng/µl (sample 1)
clone 2 : 60,5 ng/µl (sample 2)
clone 3 : 55,5 ng/µl (sample 10)
clone 4 : 56,5 ng/µl (sample 13)
PCR in linearized pSB1C3:
clone 1 : 44,0 ng/µl (sample 4)
clone 2 : 82,0 ng/µl (sample 7)
clone 3 : 77,5 ng/µl (sample 5)
clone 4 : 70,0 ng/µl (sample 14)
blue light promotor+ lacZ (ligation 6 from 1.9):
clone 1 : 79,0 ng/µl (sample 12)
clone 2 : 67,0 ng/µl (sample 9)
clone 3 : 55,5 ng/µl (sample 21)
clone 4 : 65,0 ng/µl (sample 19)
red light sensor in pSB1C3 ratio 1:3
clone 1 : 58,0 ng/µl (sample 11)
clone 2 : 52,5 ng/µl (sample 15)
clone 3 : 59,5 ng/µl (sample 8)
red light sensor in pSB1C3 ratio 1:6
clone 1 : 50,5 ng/µl (sample 18)
clone 2 : 58,5 ng/µl (sample 3)
clone 3 : 61,5 ng/µl (sample 6)
red light sensor in pSB1A3 (ligation 1:5 (from 1.9.) clone 1 : 52,5 ng/µl (sample 20)(red colony) clone 2 : 51,0 ng/µl (sample 16)
red light sensor in pSB1A3 (ligation 1:1 (from 3.9) (red colony): clone 1 : 40,5 ng/µl (sample 17)
All samples were digested except 9,21,19:
5 µl of Mini-DNA was cut using 0,5 µl E and 0,5 µl P in 2 µl NEB4 and 20 µl total volume. Digest was loaded onto 1 % agarose gel.
Red light sensor constructs look fine (3,8kbp insert and 2 kbp vector). T7ptag constructs are wrong and will be repeated (T7ptag expected at 2,6 kbp). Blue light sensor looks fine (3kbp). Red light sensor cloning into pSB1A3 didnt yield any insertion products, mainly slightly red colonies were visible --> 1kbp insert is RFP cassette. One not-red clone could be picked (sample 15), which contains a 400 bp insert of unkown origin.
--> Sample 7 was choosen to be sequenced as it yielded most amount of DNA.
[edit] Reporter Plasmid: T7 promotor prep and new RBS_LacZ prep
3 % agarose gel from yesterday was used at 100V for 1,5h
No band could be detected. Nevertheless, gel slice was cut and will be purified.
Bands were cut and purified using Wizard Kit according to manufacturer's instructions.
Concentrations was determined using Nanodrop:
T7prom E, S: 9 ng/µl
T7prom P, S: 11.5 ng/µl
RBS_lacZ E, X: 14 ng/µl
RBS_lacZ E, X: 14.5 ng/µl
[edit] 06-09-2011
People: Thorsten, Anna, Katharina
[edit] Testing
[edit] Miller Assay
5 newly inoculated cultures were assayed: difference to day before: grow in the dark (only DH5alpha)
incubation at room temperature of:
K322127 in DH5alpha in the light of lamp
K322127 in DH5alpha in the dark
K322127 in DH5alpha in the turn-off light
K322127 in BL21 in the light
cpH8 in DH5 alpha in the light
Problem 1: OD of bacterial cultures was very high, should have been around 0,7.
Problem 2: too long incubation of color reaction yielded too high ODs, samples couldn't be diluted as more blank sample (as diluent) was missing.
[edit] Cloning
[edit] Reporter Plasmid
[edit] Purification of 05-09-11 gel runs
Frozen gel slices were purified using Promega's Wizard Kit according to manufacturers instructions.
Subsequent determination of concentration via Nanodrop:
1: T7prom 46 bp cut: 9 ng/µl
2: T7prom vector cut: 11.5 ng/µl
3: RBS_lacZ vector cut: 14.0 ng/µl
4: RBS_lacZ insert cut: 14.5 ng/µl
[edit] Ligation of purified samples: Reporter Plasmid and T7ptag into shipping vector pSB1C3
Ligation mix was incubated for 1h at RT and and then at 16°C o/n.
[edit] Other Work
[edit] Sequencing of clones
Primer overview: http://partsregistry.org/Primers/Catalog
For most biobrick plasmids the standard sequencing primers VF2 (verification forward) and VR (verification reverse) will fit. They will be synthesized at GATC and stored for 1 year.
10 µl of Following clones were send for sequencing 300-100 ng/µl with VF2 primer (tgccacctgacgtctaagaa):
Bluelight_lacZ in pSB1A2 110904_clone2 miniprep conc.: 49.5 ng/µl
red light in pSB1C3 110905_sample 7 conc.: 82 ng/µl diluted 1:1 with ddH2O
[edit] 07-09-2011
People: Thorsten, Anna, Katharina
[edit] Cloning
[edit] Reporter Plasmid and T7ptag into pSB1C3
[edit] Glycogen/ethanol precipitation and transformation
- DNA precipitation with glycogen:of the ligation samples from 6-9-11
1. add 2 µl 3 M sodium acetate, 0.5 µl glycogen and 22.5 µl isopropanol to 20 µl ligation product
2. incubate the mixture at -20°C for one hour
3. centrifuge the mixture for 15 minutes at 18,000 rpm, 4°C
4. discard the supernatant
5. add 50 µl 70 % ethanol
6. centrifuge the mixture for another 10 minutes at 18,000 rpm, 4°C
7. remove supernatant with a pipet CAREFULLY. If pellet moves, centrifuge again.
8. air-dry the pellet until the ethanol is completely evaporated. Do not over-dry pellet as this makes dissolving more difficult.
9. resuspend the pellet in 10 µl nuclease-free water by pipetting carefully.
- 10 µl purified ligation was mixed with 40 µl competent cells and electroporated into DH5alpha.
- 1 ml SOC medium (SOB medium + 20 mM glucose) was used and incubation was carried out for 1h at 37°C, shaking at 500 rpm in 2 ml eppis.
- Culture was then centrifuged at 4000 rpm for 5 minutes, and resolved in 100 µl medium.
- Whole sample was plated onto LB/agar plates supplemented with appropriate antibiotic and incubated o/n @ 37°C
[edit] Synthetic construct
[edit] Digest of synthetic construct and PCR-product (in pSB1C3)
Samples were loaded on preparative gel (1 %, TAE 1x, 100 V, 2 h 30 min) and subsequently cut with a new scalpel for each sample using 365 nm illumination as short as possible. Gel slices were purified using Promega's Wizard Kit according to manufacturer's instructions. Picture was taken after cutting of gel slices in order to reduce illumination.
[edit] ligation of synthetic construct and PCR-product (in pSB1C3)
Ligation was performed at 16°C o/n
[edit] 08-09-2011
People: Anna, Katharina
[edit] Cloning
[edit] AND-Gate plasmid
- DNA precipitation with glycogen/ethanol of the ligation samples from 7-9-11 accroding to protocol 7-9-11
- transformation via electroporation of 40 µl electro competent DH5alpha cells with the full volume of purified ligation samples from 7-9-11
[edit] Reporter plasmid and T7ptag
-overnight cultures for mini prep of transformations from 7-9-2011:
four clones of plates:
2 (T7prom(2)_RBS_lacZ(4))
3 (T7prom(2)_RBS_lacZ(4))
5 (RBS_lacZ(3)_T7prom(1))
6 (pSB1C3(3)_T7pol(5))
7 (pSB1C3(3)_T7pol(5))
one clone of plate 13 (synthetic construct in delivery vector pMA-RQ)
[edit] Other work
- production of LB-Agar plates with Kanamycin (500 ml), Chloramphenicol (300 ml) and Ampicillin (500 ml)
- overnight cultures of K322127 in DH5alpha (Cam, in the dark) and cpH8 inDH5alpha (Kan) for Miller Assay the next day
[edit] 09-09-2011
People: Anna, Katharina, Thorsten
Std Primer VR seems to misprime to B0015 double terminator: http://partsregistry.org/Help:Primers/Problems_using_VF2_and_VR_to_amplify_BioBrick_parts
Could be a problem when sequencing into our construct from downstream primer binding site as we use B0015 in synthetic construct. Because sequencing was done succesfully using this primer by others, we'll try it keeping the mispriming in mind. --> B0014 would be a better terminator for next time.
pSB1C3 is using B0056 as terminator downstream of suffix.
[edit] Cloning
[edit] Reporter plasmid and T7 polymerase
- Plasmid isolation -> Miniprep of the over night cultures from 8-9-11 according to manufacturers instructions from Metabion
- measurement of the DNA concentration of the following samples:
2a: 92 ng/µl
2b: 122 ng/µl
2c: 131 ng/µl
3a: 98 ng/µl
3b: 152 ng/µl
3c: 124 ng/µl
3d: 112 ng/µl
5a: 52.5 ng/µl
5b: 55 ng/µl
5c: 74.5 ng/µl
5d: 87 ng/µl
6a: 134 ng/µl
6b: 118 ng/µl
6c: 101 ng/µl
6d: 140 ng/µl
7a: 76.5 ng/µl
7b: 113 ng/µl
7c: 83 ng/µl
7d: 71.5 ng/µl
13: 79 ng/µl
- control restriction digest of the 21 DNA samples (2a-d, 3a-d, 5a-d, 6a-d, 7a-d,13) with the restricition enzymes E,P
total volume 50 µl (39 µl nf H20, 5 µl DNA, 5 µl NEB4 Buffer, 0,5 µl of each enzyme)
incubation for 1 hour at 37°C, inactivation for 20 min at 80°C
- analytical gelelectrophoresis ( 1%, 1x TAE, 12 wells, 120 V, 1 hour 30 minutes)
50 µl DNA + 10 µl loading buffer -> 40 µl sample in each well
Size of T7promotor with RBS_lacZ looks correct (RBS_lacZ approx 3kbp pSB1AK8 approx 3.4 kbp). Ligation of 46 bp T7promotor into pSB1A2_RBS_lacZ (ID 5) didnt yield any correct clones, only vector background could be picked. Samples were discarded. Transformation of sample 4 resulted in short-circuit and was lost. Ligation of T7ptag into pSB1C3 looks fine. (T7ptag approx 2.6 kbp)
[edit] AND-Gate plasmid
transformation output: vector background looks fine
following clones have been picked:
[edit] Next steps
Besides ligation of T7ptag insert, pSB1C3_T7ptag vector will be opened to ligate with red light_synthetic construct insert--> see 10-09-11.
Send clones for sequencing on Monday.
[edit] Testing
[edit] Miller Assay
two cultures in LB medium:
K322127 in DH5alpha with three incubation conditions: lighted, dark and under off-light
cpH8 in DH5 alpha as a control
conditions of bacterial growth: 37 °C, shaken, culture K322127 in DH5alpha grown in the dark prior to the experiment
volume of bacterial suspension used: 500 µl
incubation time for Miller Assay: 10 min (37 °C)
improvement to prior experiments: start OD between 0,15 and 0,17, correct antibiotics applied, incubation time of Miller Assay only 10 min.
[edit] 10-09-2011
People: Susan, Wolfgang, Thorsten
[edit] Cloning
[edit] AND-Gate plasmid
[edit] red_light sensor - synthetic construct ligation check
- Plasmid isolation -> Miniprep of the over night cultures from 09-09-11 according to manufacturers instructions from Metabion. 4 ml culture was used and 1 ml culture was saved for potential glycerol stocks
- control restriction digest of the 21 DNA samples (2a-d, 3a-d, 5a-d, 6a-d, 7a-d,13) with the restricition enzymes E,S
total volume 10 µl (7 µl nf H20, 1 µl DNA, 1 µl NEB4 Buffer, 1 µl of each enzyme)
nuke for 1 minute at 800 W (microwave)
- analytical gelelectrophoresis ( 0.7%, 1 x TAE, 12 wells, 160 V, 20 minutes)
10 µl DNA + 2 µl loading buffer -> 12 µl sample in each well
Sample ID 3c was choosen to be used for further cloning. pSB1C3 with red light sensor and synthetic construct
Nanodrop derived DNA conc. of 3c: 114 ng/µl
samples 2, 3a, 4b and 6a were discarded
sample 7b was choosen to be synthetic construct in pSB1C3 --> sequencing on monday
[edit] last step: assembly of redlight sensor_synthetic construct in pSB1C3 with T7ptag
we took 2 approaches: ligation of T7ptag as insert and vector, respectively
[edit] Digestions
samples were loaded on 1 % preparative agarose gel and run for 1.5 h at 120V
Samples were cut (365 nm illumination as short as possible, picture taken after cutting of gel slices) and purified using Promegas Wizard purification kit according to manufacturers instructions.
[edit] Ligations
Samples were incubated o/n at 16°C.
[edit] 11-09-2011
People: Katharina, Flo, Anna
[edit] Glycogen/EtOH-precipitation of ligation samples from 10-09-11 and transformation
Put everything on ice before starting to work! Pre-cool centrifuge!
See protocol for precipitation/transformation from 07-09-11[[1]]
Changes made:
- all solutions were icecold
- pellet was air-dryed for about 10 min
- autoclaved water was used instead of nuclease-free, due to quick subsequent use in the transformation
- resuspending of the bacterial pellet after transformation was difficult (tried pipetting and vortexing), because the wrong centrifuge has been used
- 1,5 ml-Eppis were used instead of 2 ml-Eppis for the shaking step at 37°C in SOC
Samples 1, 8 and 9: comp. cells used were DH5a from 01-07-11
Samples 2 - 7: comp. cells used were DH5a from 30-08-11
Antibiotic resistance:
- 1: Amp
- 2 - 9: Cam
[edit] Testing
[edit] Inoculation of o/n-cultures for tomorrow´s Miller Assay
10 ml LB+antibiotic in 50 ml-falcon tube
add 10 ul (= 1:1000 dilution) of yesterday´s o/n-cultures:
- bluelight + lacZ in DH5a (Amp)
- redlight + lacZ (trunc.) in DH5a (Cam)
- neg. contr. Cph8 in DH5a (Kan)
Wrap falcons with aluminium foil, so growth can occur in the dark!
Shake o/n @ 37°C, 180 rpm
[edit] Miller Assay with blue light construct
two cultures in M9 medium:
blue light - lacZ on pSB1A2 in DH5alpha with two incubation conditions: lighted in blue light and dark
cpH8 in DH5 alpha as a control under room light
conditions of bacterial growth: 23,5 °C, shaken, both cultures grown under room light prior to the experiment
volume of bacterial suspension used: 0,1 ml
incubation time for Miller Assay: 5 min (37 °C)
improvement to prior experiments: start OD between 0,15 and 0,17, correct antibiotics applied
Start of Assay:
- measurement of Abs(600nm)
- new eppi with 0,5 ml of Zbuffer + 20 μl of freshly prepared 0,1% SDS+ 40 μl of Chloroform (under fume hood)
- 0,1 ml of the samples are taken and transferred to the Zbuffer
- mix the solution by vortexing for 10 s ( all samples equal vortexing time)
- transferring of 100 μl supernatant to 96 well plate (for photometer)
- initiation of assay with 20 μl of ONPG (4mg/ml) NOTE START TIME
- incubation at 37 °C
- stop reaction with 50 μl of 1 M Na2CO3 NOTE STOP TIME
- measurement of Abs (420nm) and Abs (550nm)
[edit] Results
Problems: 1. cells had a party over night: three LED (blue on, red on, red off) were on alternatively 2. did cells really grow?
[edit] 12-09-2011
People: Thorsten, Anna
[edit] Cloning
[edit] AND-Gate: Picking of colonies
Transformation and ligation efficiency of control was several thousand clones. Vector controls were lawn due to usage of CAM-plasmid contaminated electorcompetent DH5 alpha. All contaminated DH5alpha from 01-07-11 have been discarded.
3 clones of each plate have been picked and inoculated in 5 ml LB/cam o/n.
[edit] Other Work
[edit] sequencing of clones
Sequence of Blue light promotor with downstream RBS+lacZ is correct.
Sequence of first 1000 bp of PCR-Product shows one possible silent mutation in PcyA (Phycocyanobilin:ferredoxin oxidoreductase) gen on Position 45 T->C. Resulting triplet is CCC instead of CCT, which both code for proline.
Rest of PCR product should be sequenced aswell? Two more primers would be necessary to cover whole sequence.
Or: functionality check using CP919 strain should result in comparable Miller Unit output between PCR product only and red light original part K322127 as CP919 is Φ(OmpC-lacZ)10-25
[edit] Testing
[edit] Miller Assay with blue light construct
two cultures in LB medium:
blue light - lacZ on pSB1A2 in DH5alpha with three incubation conditions: lighted in room light, dark and blue light
cpH8 in DH5 alpha as a control under room light
conditions of bacterial growth: 23,5 °C, shaken, both cultures grown in the dark prior to the experiment, two hours before the experiments: cultures were subdivided and lit as described
volume of bacterial suspension used: 50 µl (or dilutions with LB medium)
incubation time for Miller Assay: 5 min (37 °C)
Start of Assay:
- measurement of Abs(600nm)
- new eppi with 0,5 ml of Zbuffer + 20 μl of freshly prepared 0,1% SDS+ 40 μl of Chloroform (under fume hood) in 2 ml tube
- 50 μl (as a dilution 25 µl + 25 µl LB medium or 10 μl + 40 μl LB medium) of the samples are taken and transferred to the Zbuffer
- mix the solution by vortexing for 10 s (all samples with equal vortexing time)
- transferring of 100 μl supernatant to 96 well plate (for photometer)
- initiation of assay with 20 μl of ONPG (4mg/ml) = START
- incubation at 37 °C
- stop reaction with 50 μl of 1 M Na2CO3 = STOP after exactely 5 min
- measurement of Abs (420nm) and Abs (550nm)
- when solution was too yellow (OD > 2.0), stopped mixtures were diluted 1 : 7 in dd H2O
[edit] Results
Problems: 1. did cells grow up to 20 h and act normally?
[edit] 13-09-2011
People: Simon, Anna, Alex, Thorsten
[edit] Other Work
[edit] Electrocompetent cells
- new electrocompetent cells (DH5alpha) were made by the following protocol:
- 20 ml LB medium was inoculated with 1 µl electrocompetent DH5alpha (kindly provided by Andrea Mückl)
- 350 ml LB medium were inoculated with 15 ml of the overnight culture and incubated at 37°C until a OD(600) of 0,7 was reached
- the medium was distributed on falcons and the cells were centrifuged at 4500 rpm for 20 min at 4°C
- after discarding the supernatant 350 ml of sterile ice-cold 10% glycerol was added and the pellets were resuspended using a pipet
- the resuspension was centrifuged at 4500 rpm at 4°C for 11 minutes
- after discarding the supernatant the cells were resuspended again in 350 ml of ice-cold 10% glycerol (cells kept chilled all the time)
- and again centrifuged at 4500 rpm at 4°C for 11 minutes
- the supernatant was discarded and the cells were resuspended in the remaining supernatant
- the cells were dispensed in 40 µl aliquots and stored at -80°C for further use
[edit] Cloning
[edit] AND-Gate
Minipreps of picked cones on 12-09-11 were done according to Metabion's instructions. Concentrations:
2a: c = 34.5 ng/µl
2b: c = 68.0 ng/µl
3a: c = 118 ng/µl
3b: c = 65.0 ng/µl
5a: c = 122 ng/µl
5b: c = 71.5 ng/µl
6a: c = 122 ng/µl
6b: c = 119 ng/µl
6c: c = 51.0 ng/µl
After restriction digest using E and P, samples were separated on a 0.8 % gel:
[edit] Vector prep pSB1A3 and pSB1C3
- The restricted vectors ID10 pSB1A3 and ID12 pSB1C3 from 30-08-2011 were applied onto a 1 % agarose gel and prepped using the Wizard SV gel purification kit.
- The vector pSB1A3 was single cut, while pSB1C3 was cut with E and P (from K322127). The expected lengths are about 2 kbp for pSB1C3 and about 3 kbp for pSB1A3, as it also contains a RFP cassette.
- The measured concentrations were 12.5 ng/µl for pSB1A3 and 11.5 ng/µl for pSB1C3, in a resuspended volume of 50 µl.
[edit] Testing
no Miller Assay today
[edit] Inoculation of o/n-cultures for tomorrow´s Miller Assay
10 ml LB+antibiotic in 50 ml-falcon tube
add 20 µl (= 1:1000 dilution) of yesterday´s o/n-cultures:
in LB medium:
- bluelight + lacZ in DH5a (Amp)
- neg. contr. Cph8 in DH5a (Kan)
in M9 medium:
- redlight + lacZ (trunc.) in DH5a (Cam)
- neg. contr. Cph8 in DH5a (Kan)
Wrap falcons with aluminium foil, so growth can occur in the dark!
Shake o/n @ 37°C, 180 rpm
[edit] Other Work
1. preparation of Zbuffer and Na2CO3 solution for next day
2. Transformations:
- chemical transformation of pSB1AK8 T7prom RBS lacZ (plasmid from clone 2c, 09.09.2011) in BL21 DE3. The BL21 cells we received from Lars Mitschke turned out to be chemically competent cells. Transformation was performed based on a protocol from openwetware.org. For detailed description of the protocoll see Methods.
- electroporation of Part R2 (= RBS + lacZ) in pSB1A2 using today's and the old electrocompetent DH5alpha cells.
[edit] Sequencing results
pSB1AK8_T7prom_RBS_lacZ_VF2_110909_clone_2c-GATC-VF2-545457: Sequence shows one missing T in scar between T7promoter and RBS of lacZ. Rest of sequence is fine.
Sequence
 "
"