Team:SJTU-BioX-Shanghai/Project/Subproject2
From 2011.igem.org
(Difference between revisions)
(→Introduction) |
(→Background) |
||
| (32 intermediate revisions not shown) | |||
| Line 15: | Line 15: | ||
-------- | -------- | ||
| - | The Rare Codon | + | The Rare Codon Switch can only regulate the amount of target protein production. To make our device a strict molecular switch that turns on/off protein biosynthesis, we need to eliminate the background. |
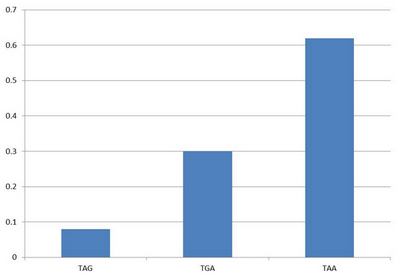
| - | In recent years, stop codons (codon<sub>ST</sub>) and stop codon suppressor tRNAs (tRNA<sub>SS</sub>) are used to incorporate unnatural amino acids in protein biosynthesis. Peter Schultz and | + | In recent years, stop codons (codon<sub>ST</sub>) and stop codon suppressor tRNAs (tRNA<sub>SS</sub>) are used to incorporate unnatural amino acids in protein biosynthesis. Researchers like Peter Schultz and Kiga have made a lot of contributions in this field. The advantage of codon<sub>ST</sub> and tRNA<sub>SS</sub> is that they can incorporate target amino acids without background noise. We make use of codon<sub>ST</sub> and tRNA<sub>SS</sub> as the controlling element for protein biosynthesis. In ''E.coli'', the rarest used stop codon is TAG. |
| + | |||
| + | [[image:11SJTU_overview_1.jpg|center|frame|''Fig.1'' stop codon usage frequency in ''E.coli'']] | ||
===Introduction=== | ===Introduction=== | ||
| Line 27: | Line 29: | ||
We can control the translation process by controlling whether the ribosome can get through the stop codon placed in the target protein's mRNA. | We can control the translation process by controlling whether the ribosome can get through the stop codon placed in the target protein's mRNA. | ||
| - | This process can be achieved by controlling the existence of charged | + | This process can be achieved by controlling the existence of charged tRNA<sub>SS</sub> that recognizes the stop codon. This process is controlled by two elements: |
| - | The existence of | + | The existence of '''tRNA<sub>SS</sub>''' |
| - | + | '''aaRS''' that can charge tRNA<sub>SS</sub> | |
| - | Reporter: a stop codon is put immediately after the initial codon ATG in the target protein' s mRNA. | + | Reporter: '''a stop codon''' is put immediately after the initial codon ATG in the target protein' s mRNA. |
===Design=== | ===Design=== | ||
| Line 39: | Line 41: | ||
-------- | -------- | ||
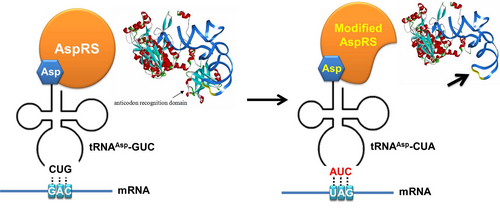
| - | + | *tRNA<sup>Asp</sup>-TAG: tRNA<sup>Asp</sup>-TAG with its anticodon mutated to CUA can base pair with stop codon UAG. | |
| - | * | + | *aaRS: the modified AspRS without anticodon recognition domain. This modified enzyme can charge Asp to tRNA<sup>Asp</sup>-TAG. |
| - | |||
| - | + | [[image:11SJTU_stop_00.jpg|center]] | |
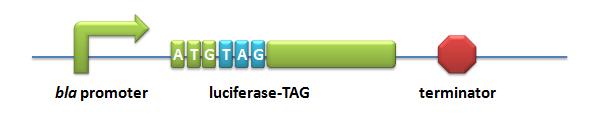
| - | + | *Luciferase-TAG: A stop codon TAG is put immediately after the start codon ATG in the luciferase gene. | |
| - | + | [[image:11SJTU-LUC-TAG.jpg|Pbla-Luc-TAG]] | |
| - | + | ||
| - | + | ===Action=== | |
| - | + | -------- | |
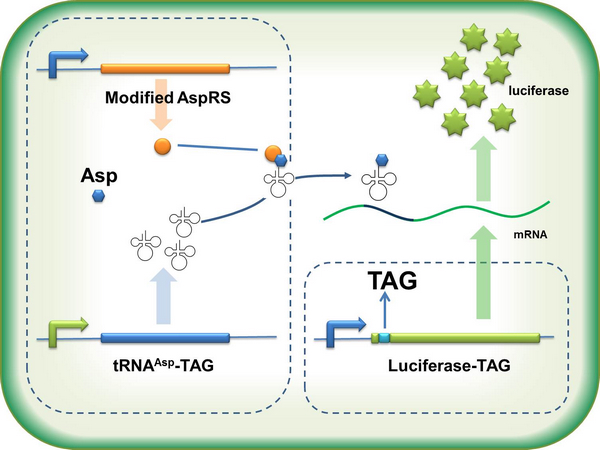
| - | + | If the ribosome can get through the stop codon with the help of Stop-Codon Switch, luciferase can be expressed. Otherwise, luciferase cannot be expressed. | |
| - | + | [[image:11SJTU_stop_01.jpg|center]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[image: | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
===Results=== | ===Results=== | ||
------------- | ------------- | ||
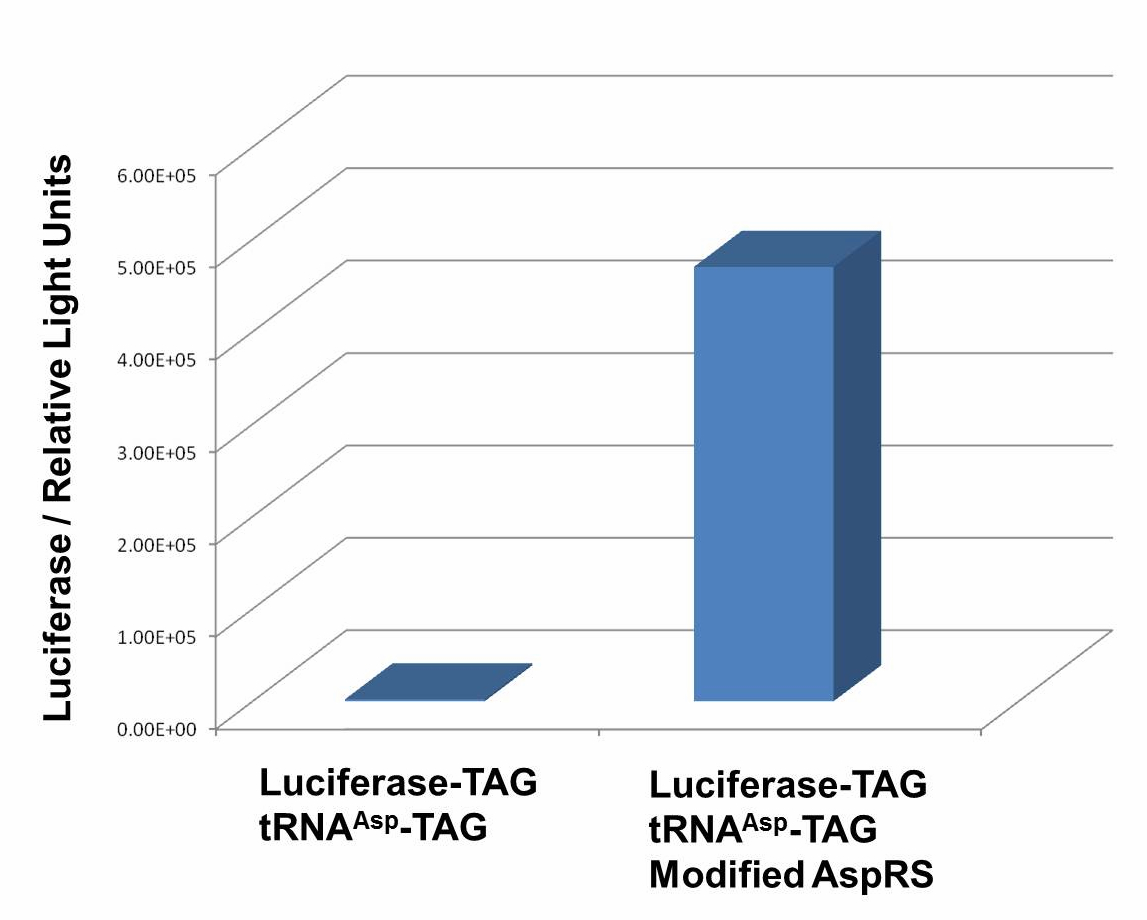
| - | + | We have used P''bla''-Luc-TAG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567003 BBa_K567003]) as our Reporter. The amount of luciferase produced is reflected using the bioluminescence emitted during the luciferin reaction. Our results demonstrate that TAG insertion into luciferase blocks luciferase production, which was shown in the control group. In the experimental group, with the help of PT7-TDRS([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567011 BBa_K567011]) and tRNA<sup>Asp</sup>-TAG([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567013 BBa_K567013]),luciferase was produced and bioluminescence was emitted during the luciferin reaction. These results proved that '''Stop-Codon Switch can turn on protein expression.''' Yet further work is needed to optimize the device. | |
| - | + | [[image:11SJTU_stop_02.jpg|thumb|600px|center|''Fig.1'' Functional Analysis of Stop-Codon Switch. ER2566 cannot produce luciferase with Pbla-Luc-TAG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567003 BBa_K567003]) only. When PT7-TDRS ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567011 BBa_K567011]) and tRNA<sup>Asp</sup>-TAG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567013 BBa_K567013]) are also transformed into the cell, luciferase is produced. The results proved Stop-Codon Switch as a strict molecular switch without background noise.]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[image: | + | |
===Conclusions=== | ===Conclusions=== | ||
------------- | ------------- | ||
| - | We have successfully constructed the Stop-Codon Switch | + | We have successfully constructed the Stop-Codon Switch and have tested the device. Results proved Stop-Codon Switch as a strict molecular switch without background noise. tRNA<sup>Asp</sup>-TAG recognizes stop codon TAG and can be charged with Asp by modified AspRS. |
| - | + | ||
===Referance=== | ===Referance=== | ||
Latest revision as of 02:49, 29 October 2011
 "
"