Team:Potsdam Bioware/Safety Ethics
From 2011.igem.org
m (→Safety & Ethics) |
(→Safety & Ethics) |
||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
== Safety & Ethics == | == Safety & Ethics == | ||
| + | <br> | ||
== Safety Assesment == | == Safety Assesment == | ||
Revision as of 14:13, 17 September 2011
Safety & Ethics
Safety Assesment
Our iGEM project requires only the handling of the non-pathogenic, non-adherent Escherichia coli K12 and B strains and the well-established filamentous phage. Both, the bacteria and the phage are commonly used chassis in laboratories and pose no risk when handled according to the mandatory rules. As all of us are well briefed about laboratory safety and biohazard regulations we follow these at all times. In Germany, work with genetically modified organisms is regulated by the ‘Law on Gene Technology’ (Gesetz zur Regelung der Gentechnik, GenTG). According to these rules, the responsible governmental authorities of the state of Brandenburg have been notified about our work. Following these rules, there should not be a significant danger neither to the environment nor to team members.
The most important issues we discussed are the consequences of the error-prone PCR we use to modify our parts. We tried to estimate the chances of generating highly toxic proteins. Surveying the literature, we found several reports about natural variants of microviridins and one rational mutational study, but no reports on toxic effects. As cyanobacteria can also produce toxic compounds (non-ribosomal peptides named microcystins) toxicity testing is well established in the cyanobacteria research community, and obviously, testing did not identify toxic effects. Therefore, we assume that our mutations will not have any hazardous effects. Additionally, the obtained, constructed, and planned plasmids contain only previously described parts without any known risk potential. Therefore, as far as we can foresee, our constructed BioBricks will not have or trigger any toxic effects or be critical in any way for the environment. This means that only a negligible risk arises from our used methods and constructs to the environment, the public and the team members. Last but not least, we do not see any particular danger of abuse or other security threat of our work, since it is specifically addresses scientific questions. It is our goal that the health of mankind and the environment benefit from our research.
Safety Questions
- Would any of your project ideas raise safety issues in terms of: researcher safety, public safety, or environmental safety?
- No, our project is not raising any of these safety issues.
- Do any of the new BioBrick parts (or devices) that you made this year raise any safety issues? If yes, did you document these issues in the Registry? how did you manage to handle the safety issue? How could other teams learn from your experience?
- No, our BioBrick parts or devices are not going to raise any safety issues.
- Is there a local biosafety group, committee, or review board at your institution? If yes, what does your local biosafety group think about your project? If no, which specific biosafety rules or guidelines do you have to consider in your country?
- In Germany, work with genetically modified organisms is regulated by the ‘Law on Gene Technology’ (Gesetz zur Regelung der Gentechnik, GenTG). According to these rules, the responsible governmental authorities of the state of Brandenburg have been notified about our work. Our work was classified as biosafety level 1.
- Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?
- So far biosafety assessment is primarily based on the characterization of wild-type devices or systems. For Synthetic Biology these rules should be extended to better represent the variations made by synthetic biology approaches. In addition to the continuous evaluation of safety and security, a section on technological impact assessment should be added.
Ethics
Why Ethics?
Synthetic Biology is not only a current topic in the science community. A look through published articles in papers e.g. the Guardian, abstracts from governmental
institutions and a large amount of webblogs regarding the latest developments in this new field, strengthen the impression of a general interest.
The reasons for this development are sure a combination of different factors. With the possibilities of the modern web and media people have the chance to
understand science and communicate about new results. A general interest for information is forming with a new generation. The typical image of a researcher in a
ivory tower has been replaced by lighthouses of knowledge, which interact with the society.
Former mistakes, where few decided in which amount and time manner new results in research are used e.g. the usage of nuclear power, are not imaginable in the
age of information. These social trends forces science to take on a dialogue with society about the use of new research results, application and commercialization.
We tried to think about these certain issues by holding a lecture on this topic, resulting into a field of forces for Synthetic Biology and a survey to reflect the opinion
of the German politics.
Seminar: "From engineer to creator: a controversy"
On the 5. July the iGEM Team Potsdam hosted a lecture about
Ethics in the field of Synthetic Biology. As guest speaker we were
able to welcome Prof. Dr. Ralf Stoecker, professor of philosophy
at the University of Potsdam and also member of the board of
the Academy for Ethics in Medicine.
This lecture was not only for the iGEM Team itself, but for all
students with different field of study.
Through advertisement in different departments a audience with
different background was the result.
Starting with a general overview in the history of philosophy
regarding the metaphysics of morals and the example of
Immanuel Kant, Prof. Dr. Stoecker tried to explain the difficulty
of ethics in modern times.
Kant started to think about general ideas concerning actions of humans a priori. Philosopher often try to
adapt these moral principles to certain specific cases. Nowadays they are confronted with the problem of a
fast development in science and new applications. It is not any more possible to think first about a general
principle and then adapt these to the new results. Today the society often needs an answer to a moral
problem in short time, because the applications are needed or will be commercialize anyway. One can then
try, to extract from the concrete problem e.g. Synthetic Biology in medicine general principles. This way is part
of the applied philosophy.
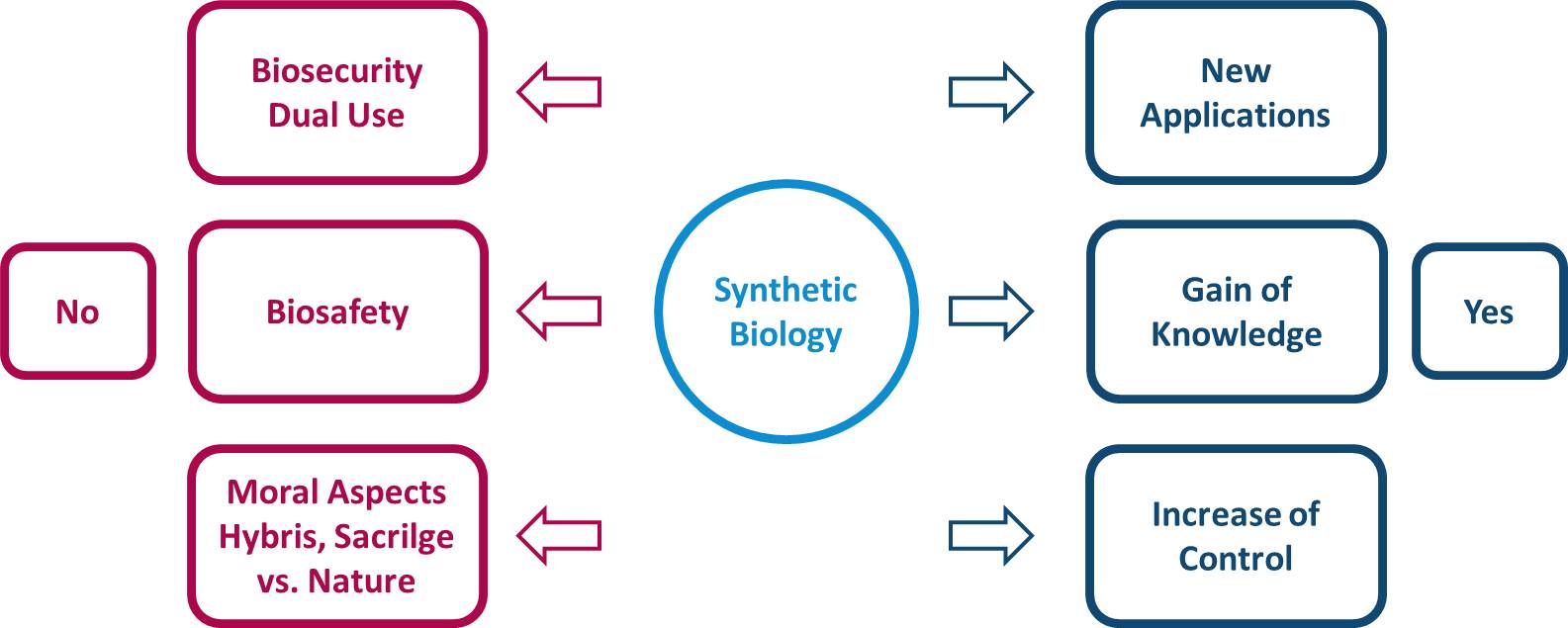
To represent this current problem in finding a ethic view on Synthetic Biology, Prof. Dr. Stoecker suggested a
field of forces, where different positive and negative effects pull at the decision to use Synthetic Biology in
research for new applications. In conclusion, the ethic or moral decision for a new case in not determined by
higher general principles. It is a combination and weighing of different positive and negative aspects.
We tried to find relative clear examples for advantages and disadvantages of Synthetic Biology. One should always remind himself, that the different aspects have different weight for each individual. While the iGEM Competition is a good example for advantages of Synthetic Biology e.g. new applications and teaching of new knowledge, Miller and Selgelid5 show in their Paper, that new applications in biology also can used for military use . The „lone operator” scenario by Tucker and Zilinkas6 is also a good example for possible biosafety problems. In this unlikely scenario one professional researcher follows his own terrorist goal. Still these examples show disadvantages which one has to consider, when trying to make his decision.
 "
"