Team:Potsdam Bioware/Project/Summary
From 2011.igem.org
(→Microviridin) |
(→Microviridin) |
||
| Line 31: | Line 31: | ||
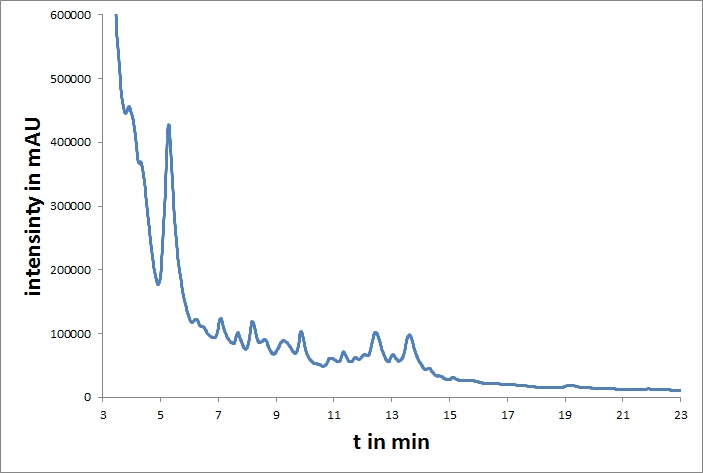
[[File:UP_HPLC_mvd_01.jpg|left|300px|thumb|'''Figure 1:''' HPLC chromatogram of mdnA]] | [[File:UP_HPLC_mvd_01.jpg|left|300px|thumb|'''Figure 1:''' HPLC chromatogram of mdnA]] | ||
<br> | <br> | ||
| - | + | ||
The microviridin sub-project aimed for modifications at the genetic level such that protease inhibiting activities of the resulting peptides are enhanced. We used random mutagenesis or focused randomization of oligonucleotides for the creation of gene libraries, which can be screened for mdnA-variants with therapeutically promising mutations. For further experiments, we also fused mdnA to a myc-tag.<br> | The microviridin sub-project aimed for modifications at the genetic level such that protease inhibiting activities of the resulting peptides are enhanced. We used random mutagenesis or focused randomization of oligonucleotides for the creation of gene libraries, which can be screened for mdnA-variants with therapeutically promising mutations. For further experiments, we also fused mdnA to a myc-tag.<br> | ||
In addition, all coding genes of the mdn-cluster were converted to BioBricks. The microviridin from mdnA was purified and characterized by HPLC and cyclization was verified by mass spectrometry.<br> | In addition, all coding genes of the mdn-cluster were converted to BioBricks. The microviridin from mdnA was purified and characterized by HPLC and cyclization was verified by mass spectrometry.<br> | ||
For the quick expression of several library clones, we also built auxiliary plasmid backbones with inducible promoters according to a proposed extension of the iGEM cloning standard. | For the quick expression of several library clones, we also built auxiliary plasmid backbones with inducible promoters according to a proposed extension of the iGEM cloning standard. | ||
'''[[:Team:Potsdam_Bioware/Project/Details_Microviridin|[more]]]''' | '''[[:Team:Potsdam_Bioware/Project/Details_Microviridin|[more]]]''' | ||
| + | <br> | ||
<br> | <br> | ||
<br> | <br> | ||
Revision as of 23:26, 28 October 2011
Summary
Modification, Selection and Production of Cyclic Peptides for Therapy
One key task of biopharmaceuticals is the binding and blocking of deregulated proteins. Picking the right lead structure for biopharmaceuticals is very important for success. This year, we developed a novel system for the modification, selection and optimization of peptides showing potential for protease inhibition. These promising inhibitors are found in cyanobacteria and are called Microviridins. They belong to a class of peptides characterized by unusual ω-ester and ω-amide bonds between the amino-acid side chains, which are also referred to as depsipeptides. These modifications are introduced post translationally by a set of enzymes and result in an extraordinary tricyclic cage structure.
Proteases are a large enzyme family and comprise about 647 human gene products. Therefore they are important drug targets. In addition, many harmful bacteria, viruses and fungi use proteases in their reproduction cycle and growth. The ability to block these proteases is highly relevant for therapy. Prominent examples of targets are the angiotensin-converting enzyme (ACE) and HIV proteases.
We chose a 6.5 kb Microviridin gene cluster named mdnABCDE from Mycrocystis aeruginosa NIES843. The mdn gene cluster comprises (i) a gene encoding a precursor peptide named MdnA, which is then modified to form the Microviridin, (ii) two genes encoding ATP-grasp-type ligases named mdnB and mdnC, (iii) an ABC- transporter encoding gene named mdnE as well as (iv) one gene encoding an N- acetyltransferase of the GNAT family named mdnD (Ziemert et al., 2008). Our major aim was to modify the MdnA precursor peptide and optimize its protease inhibiting properties. Towards this goal, we synthesized semi-rational mdnA gene libraries with partially randomized oligonucleotides. One library was cloned, verified by sequencing, and successfully used for selection.
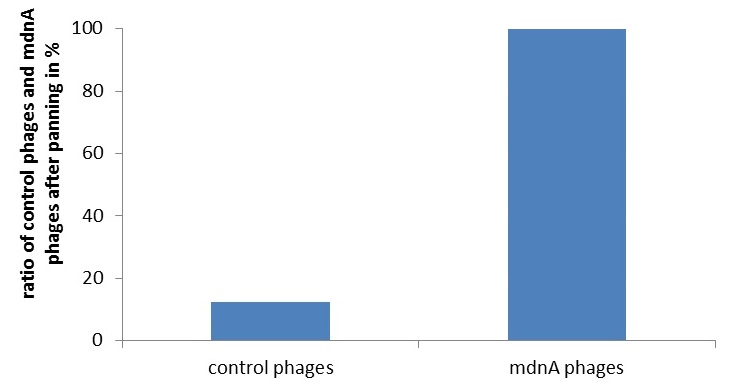
To identify the best candidates inhibiting various given proteases, we established two different selection systems: Phage Display and a new developed in-vivo selection assay. Phage display is a frequently used technique in laboratories, yet to our knowledge, phage display of cellularly cyclized peptides is new. We constructed a fusion between the surface protein geneIII of the phage and the mdnA gene within the mdnA gene cluster. This system was verified by western blotting, Phage-ELISA and controlled phage panning experiments.
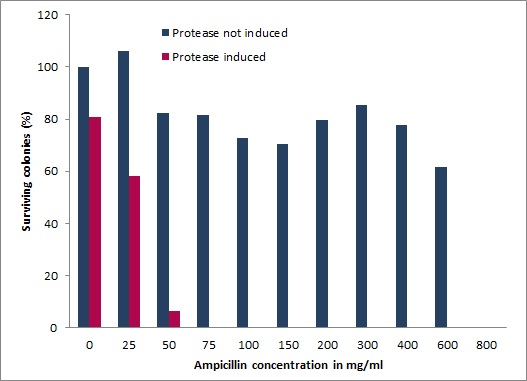
We also devised and constructed a recombinant in-vivo selection system linking protease degradation to antibiotic resistance. This system is divided into three parts: first a protease activity detector device, second a protease generator device, and third a protease blocking device. For the protease activity detector device we fused various protease cleavage sites between a signal sequence and the antibiotic resistance conferring enzyme β-lactamase. β-lactamase confers only resistance when transferred to the periplasm of E. coli. Thus, when the protease cleaves the signal sequence from the lactamase antibiotic resistance is abolished and cells die under selective pressure. However, when our Microviridin variants inhibit the protease, cells survive, allowing for an easy and efficient selection of protease inhibitors. We demonstrated a wide dynamic range of the system between expressed and non-expressed protease in the presence of increasing ampicillin concentrations. Importantly, after co-transformation with our mdnA-libary we were able to isolate several clones, which are currently being characterized.
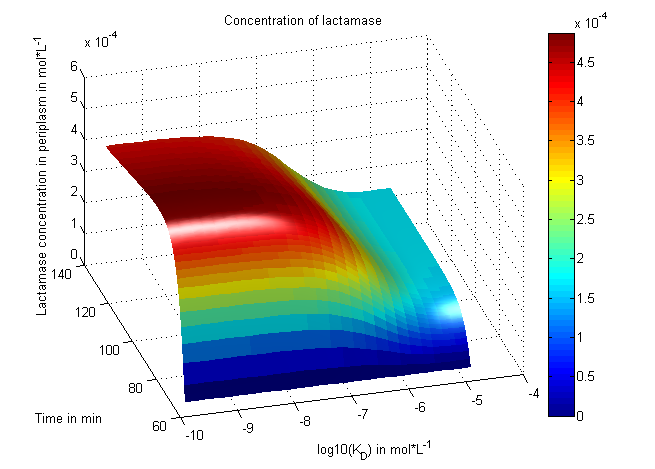
In addition to the practical work, we established a mathematical model of the in-vivo selection system. This model, which we were able to fit to experimental data, helped us to understand the selection process. We modeled the reaction kinetics with ordinary differential equations and simulated and fitted data with matlab.
Last but not least, we had several human practice projects. We send a survey to all members of the German parliament, visited a member of the parliament, held seminars on ethics and invited children to the lab.
- Ziemert, N., Ishida, K., Liaimer, A., Hertweck, C. & Dittmann, E. (2008). Ribosomal synthesis of tricyclic depsipeptides in bloom-forming cyanobacteria. Angewandte Chemie (International ed. in English) 47, 7756-9
Highlights
Microviridin
The microviridin sub-project aimed for modifications at the genetic level such that protease inhibiting activities of the resulting peptides are enhanced. We used random mutagenesis or focused randomization of oligonucleotides for the creation of gene libraries, which can be screened for mdnA-variants with therapeutically promising mutations. For further experiments, we also fused mdnA to a myc-tag.
In addition, all coding genes of the mdn-cluster were converted to BioBricks. The microviridin from mdnA was purified and characterized by HPLC and cyclization was verified by mass spectrometry.
For the quick expression of several library clones, we also built auxiliary plasmid backbones with inducible promoters according to a proposed extension of the iGEM cloning standard.
[more]
Phage Display
Phage display is a powerful tool for selecting peptides or proteins that bind and regulate the function of target proteins. It is defined as a system in which the protein and its encoding gene are covalently linked. Because of the therapeutic interest of microviridins as protease inhibitors a selection system for screening recombinant mdnA-libraries is of great importance. In our project the fundamental suitability of phage display for this purpose was shown. Therefore an appropriate phagemid, carrying an mdnA-myc-geneIII fusion gene, was constructed. The expression of the mdnA-myc-geneIII protein in the cells was shown by western blotting. Furthermore the production of phage particles carrying MdnA was determined by ELISA and phage display. [more]
In Vivo Selection
In addition to the Phage Display we developed a novel selection system. The design aimed for a cheap and time-saving alternative in contrast to an in vitro screen of protease inhibition kinetics. The assay allows us to select effective inhibitors for a random protease, among the billions of randomly generated mutants of the Microviridin. For this purpose we designed a plasmid containing two devices, first a protease activity detector and second a protease generator.[more]
Modeling
There is no synthetic biology without modeling, of course. We focused on systems modeling of our invivo selection system in which the reaction kinetics are analyzed and outcomes are predicted. Thus a synthetic biology approach can be chosen because a better understanding of the system is achieved and further changes can be planed - just like in engineering.
The reactions in our system were written down as equations under consideration of their induction at different times and the substance concentrations were numerically propagated through time. Using our concentration calculations we were able to see that our system works very well in theory - it is robust against changes of the most important system parameters. We learned about correct time-scales for our triggering and we were able to identify expected cell-division rates as a reference for the lab work. In a final step we were able to fit our model to wet-lab measurements so that predictions are more reliable. [more]
Ethics
For information about an ethics seminar and a survey among politicians see [here].
Software
Have a look at the features and screenshots of our BioLog app on this [page].
 "
"