Team:Potsdam Bioware
From 2011.igem.org
Production, Evolutionary Modification, and Selection of Cyclic Peptides for Therapy
Abstract
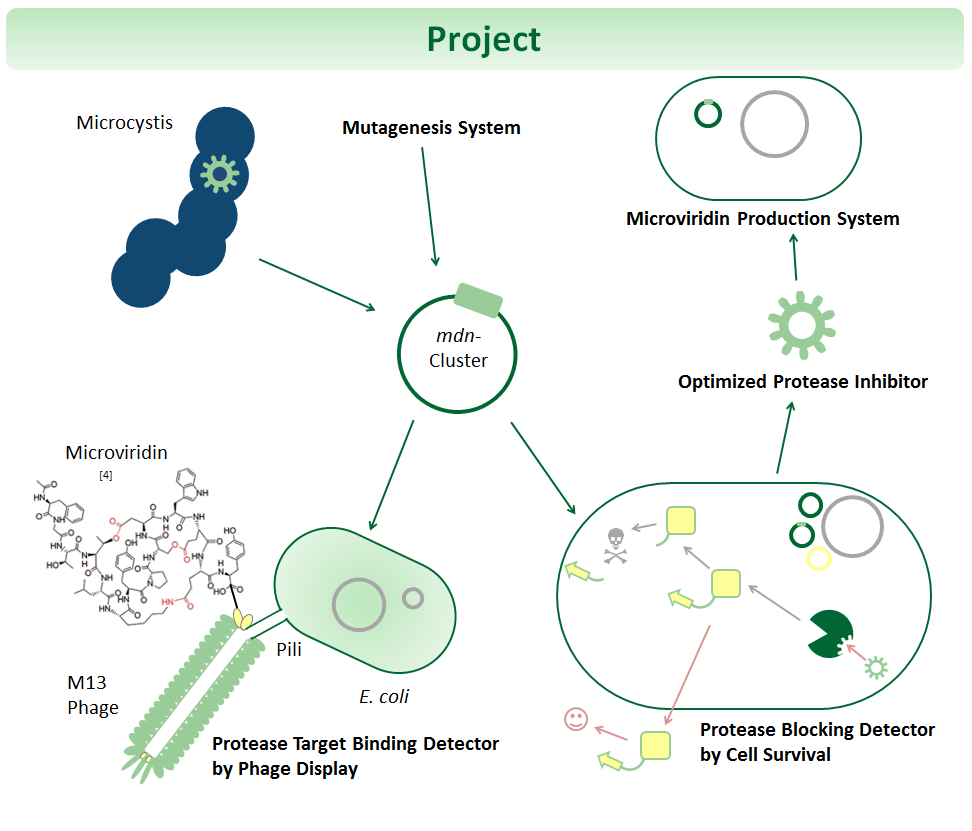
One of the most important tasks of biopharmaceuticals is the binding and blocking of deregulated proteins. Towards this goal, we mutate and select microviridins, which are tricyclic depsipeptides from cyanobacteria. They are small but stable due to their post- translational side-chain crosslinking and have high therapeutic potential for e.g. blocking proteases linked to diseases. Yet the possibilities of cyclic peptides are largely untapped since genetic systems for evolutionary optimization are not existent or not well established. Thus, we are developing modular systems for the production, mutation, and selection of such peptides. For production, we use the 6.5 kilo base mdn gene cluster cloned in E. coli plasmids. For mutation, we apply error prone PCR in combination with efficient cloning. For selection, we are establishing and testing phage display as well as an in vivo selection device, which links blocking of protease activity to antibiotic resistance. All systems, including the 6.5 kilo base cluster, adhere to the BioBrick standards.
Safety and Security Assessment
Our iGEM project requires only the handling of the non-pathogenic, non-adherent Escherichia coli K12 and B strains and the well-established filamentous phage. Both, the bacteria and the phage are commonly used chassis in laboratories and pose no risk when handled according to the mandatory rules. As all of us are well briefed about laboratory safety and biohazard regulations we follow these at all times. In Germany, work with genetically modified organisms is regulated by the ‘Law on Gene Technology’ (Gesetz zur Regelung der Gentechnik, GenTG). According to these rules, the responsible governmental authorities of the state of Brandenburg have been notified about our work. Following these rules, there should not be a significant danger neither to the environment nor to team members.
The most important issues we discussed are the consequences of the error-prone PCR we use to modify our parts. We tried to estimate the chances of generating highly toxic proteins. Surveying the literature, we found several reports about natural variants of microviridins and one rational mutational study, but no reports on toxic effects. As cyanobacteria can also produce toxic compounds (non-ribosomal peptides named microcystins) toxicity testing is well established in the cyanobacteria research community, and obviously, testing did not identify toxic effects. Therefore, we assume that our mutations will not have any hazardous effects. Additionally, the obtained, constructed, and planned plasmids contain only previously described parts without any known risk potential. Therefore, as far as we can foresee, our constructed BioBricks will not have or trigger any toxic effects or be critical in any way for the environment. This means that only a negligible risk arises from our used methods and constructs to the environment, the public and the team memberes.Last but not least, we do not see any particular danger of abuse or other security threat of our work, since it is specifically addresses scientific questions. It is our goal that the health of mankind and the environment benefit from our research.
 "
"