Team:Paris Liliane Bettencourt/Notebook/2011/07/30/
From 2011.igem.org
(Created page with "== Cyrille == === QCM on the pHM3 plasmid === The products of the miniprep done the day before was digested with EcoRI frast digest. A tube with 1 mug of DNA, 1 muL of 10x Fas...") |
(→Putting in culture Lac-::Kan strains for test) |
||
| Line 13: | Line 13: | ||
The experiment will be done again with different concentrations of primers on Monday | The experiment will be done again with different concentrations of primers on Monday | ||
| - | === Putting in culture Lac-::Kan strains for | + | === Putting in culture Lac-::Kan strains for glycerols === |
2x [ 10 mL of LB + 10muL of Kan + 1 colonie ] was prepared and grow overnight for glycerols | 2x [ 10 mL of LB + 10muL of Kan + 1 colonie ] was prepared and grow overnight for glycerols | ||
Revision as of 18:52, 30 July 2011
Contents |
Cyrille
QCM on the pHM3 plasmid
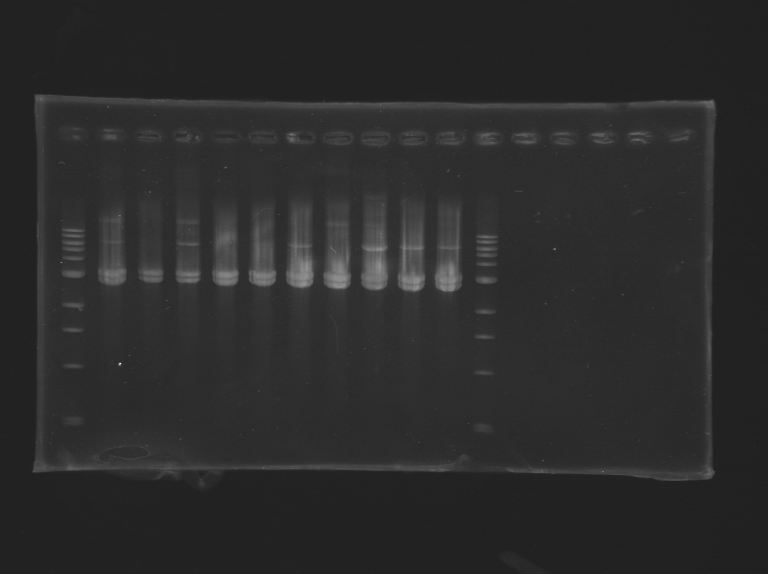
The products of the miniprep done the day before was digested with EcoRI frast digest. A tube with 1 mug of DNA, 1 muL of 10x Fast gigest grenn buffer, 1muL of enzyme qsp 10 muL with water was prepared and digested at 37°C for 15 min. The result was loaded on the gel with DNA ladder. The results are the following:
All the tubes present 2 bands at 3 kb, showing the plasmid was digested at both restriction site. This result shown that none of the PCR have worked until the end. There is suspicion about the turbocells: is there the need for a ligation done by the cell or not? Is there a problem of tranformation of the PCR plasmids regarding to the metylated plasmid?
The experiment will be done again with different concentrations of primers on Monday
Putting in culture Lac-::Kan strains for glycerols
2x [ 10 mL of LB + 10muL of Kan + 1 colonie ] was prepared and grow overnight for glycerols
Test the previously prepared MH1 competent cells
Two kind of experiments where carried out.
From 3 randomly choosen tubes in the freezer, 2 aliquots of 100 muL where made. One will be transformed with a RFP plasmid (S27). The second will undergo a transformation process but without extra plasmid.
- Aliquots 1, 2, 3 from tubes 1, 2, 3 was transformed with S27 by the heat chock process. ( 4°C 30min, 42°C 1min, 4°C 5 min ) + 1 mL LB for 2h. Then 100muL and 500muL where plated on cloranphenicol plates.
- Aliquots LB, A, K, Cm from tubes 1, 1, 2, 3 resp. had undorgone the same transformation process but without DNA added in the tube. 500 muL of each tube was then plated resp. on LB, Ampicilyn, Kanamicyn, Cloranphenicol plates. These last three are expected to dye. This is a control to see whereas the cells has survived the competence process, and if, under the stress, they have not incorporated any plasmid that would disturb further experiments.
Results will be readed tomorrow.
 "
"