Team:Paris Bettencourt/Project
From 2011.igem.org
(→GFP-LacO construct) |
(→GFP-LacO construct) |
||
| Line 61: | Line 61: | ||
The aim is to focalise diffused fluorescence on few spots to be visible. <br> | The aim is to focalise diffused fluorescence on few spots to be visible. <br> | ||
| - | We use recombinant fusion protein GFP:LacI and YFP:tetR | + | We use two differents B. Subtilis strains : |
| + | *One with recombinant fusion protein GFP:LacI (plasmid pSG20) and YFP:tetR (plasmid pFX234) | ||
| + | *One with mini-F plasmids containing lacO / tetR array. | ||
| + | |||
| + | GFP:LacI and YFP:tetR diffuse and bind on lacO / tetR array. | ||
===Characterization of the nanotubes=== | ===Characterization of the nanotubes=== | ||
Revision as of 09:40, 8 July 2011
| Home | Team | Official Team Profile | Project | Designs | Parts Submitted to the Registry | Modeling | Notebook | Safety | Attributions |
|---|
Contents |
Overall project
We started to work on one of the most intriguing microbiology discovery of the last decade: the existence of nanotubes in Bacillus subtilis!
Summary:
We decided to take advantage of an article published by Dubey and Ben-Yehuda [http://bms.ucsf.edu/sites/ucsf-bms.ixm.ca/files/marjordan_06022011.pdf] in the Journal Cell where they show an extraordinary new form of communication between Bacillus subtilis cells and even exchanges with E. coli.
The existence of the nanotube network discovered by Dubey and Ben-Yehuda is still discussed. We wanted to use synthetic biology to provide new evidences supporting the existence of a new cell-to-cell communication in Bacillus Subtilis and between Bacillus Subtilis and E.coli. We also aim at proposing new applications combining synthetic biology and the nanotubes network.
Steps of the project:
Direct observation
First, we want to prove de novo what the authors found. Although some microscopy images prove solidly the existence of these so-called nanotubes, we aim at using synthetic biology to get a definite proof of the existence of nanotubes. We will simply re-do the experiments done in the paper with simple observations.
Designs for direct observation step.
Characterization
Our second aim is to characterize the nanotubes: what passes through them and what are the typical diffusion times through the network. We will examine if RNA, proteins of different sizes and/or metabolites can pass through and with which ease and rate. For that purpose, we are going to engineer, thanks to Synthetic Biology, [http://en.wikipedia.org/wiki/BioBrick BioBricks] following the general design: an emitter cell that would produce a messenger (RNA, protein etc.) that would then pass through the nanotubes and into the receiver cell. The latter, would then have specific promoters that would induce an amplification system that would in turn trigger a detection mechanism (fluroescence, others). As a general outline we will first investigate the inter-species (subtilis-coli) connection thanks to all the existing biobricks for E .coli then we will move on to an intra-species (subtilis to subtilis) connection and develop new parts specific to subtilis.
Designs for nanotube characterization.
Master-slave system
A very intersting system would involve a master strain and a slave strain. The master would have complete control over the state of the slave. Toggle switches seem to be an excellent idea to obtain this kind of behaviour.
Designs for a Master-Slave system.
Bi-directional communication
There are other goals that we are still working on such as use of this nanotube network to perform more complex tasks (pattern formation for instance) using more complex genetic circuits. This constructs would probably involve some kind of bi-directionnal communication, each cells reacting to the behaviour of its direct neighbours.
Designs for a bidirectional communication.
Project Details
Direct observation
Proof of principle: the first step is about reproducing what the authors of the original paper already did, to make sure that we can obtain the same results.
Antibiotics
It has been shown that the nanotubes allow for a transient antibiotic resistance. In order to prove this part, we use two different strains of subtilis. Each strain has a different antibiotic resistance encoded and when grown together on a double selective medium colonies still form. The limitations of this simple method is that we don't know if both strains survive or only one does. Different hypothesis follow from there: either both survive because both antibiotics are exchanged or one strain only survives because one antibiotic preferentially get through the nanotubes.
Simple GFP
Once again, we will re-create an experiment undertaken by the original authors. It consists in mixing two different strains of subtilis: one is gfp+ and one is gfp-. Using an overlay of phase contrast microscopy and fluorescent microscopy we shoud be able to see which strain is which and also observe the formation of a GFP gradient. This will be a stronger statement towards the existence of the nanotubes.
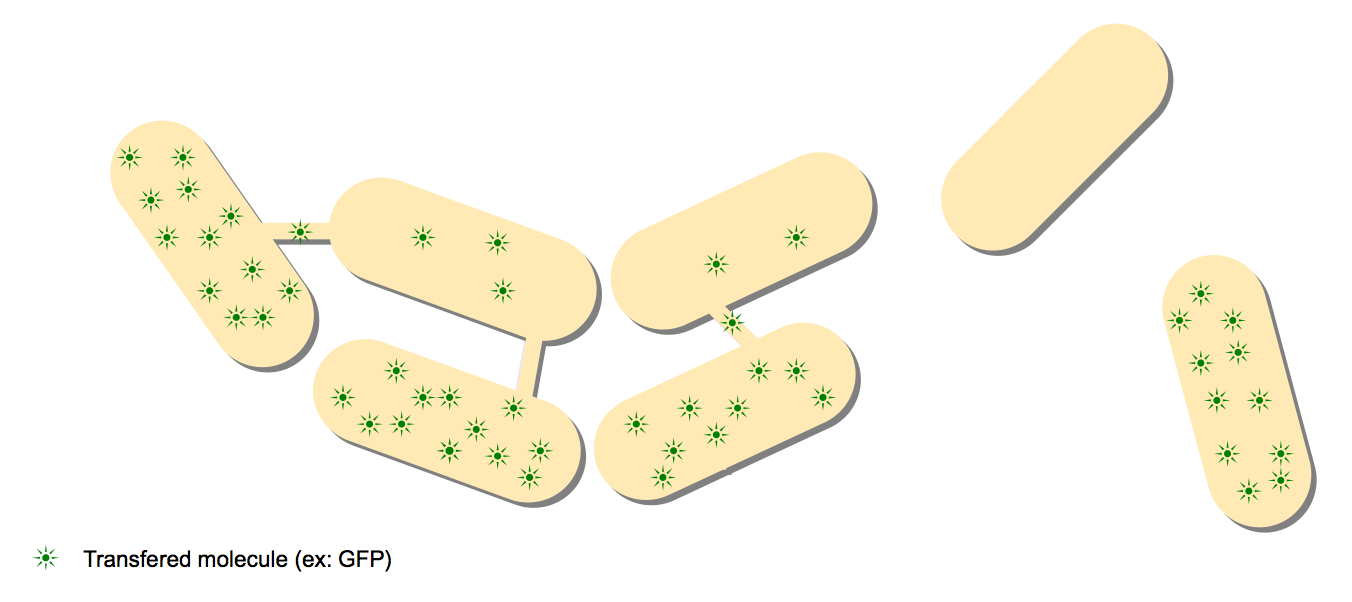
GFP-LacO construct
The aim is to focalise diffused fluorescence on few spots to be visible.
We use two differents B. Subtilis strains :
- One with recombinant fusion protein GFP:LacI (plasmid pSG20) and YFP:tetR (plasmid pFX234)
- One with mini-F plasmids containing lacO / tetR array.
GFP:LacI and YFP:tetR diffuse and bind on lacO / tetR array.
Characterization of the nanotubes
Master-slave system
Master and slave relationships aka irreversible systems: at this point we will have re-proven the existence of the nanotubes and will know how to correctly manipulate B. subtilis, it is now time to use the BioBricks that were sent to us. In this step, we shall using two different kinds of strains. As mentionned in the title of the step, there will be a master which is actually the signal emitter cell and there will be a slave which will be the receiver cell.
Bi-directional system
Sponsors
Logo Fondation Bettencourt Schueller.gif
[http://www.fondationbs.org Bettencourt Foundation (in French)] |
Geneious Logo.gif
[http://www.geneious.com/ Geneious] |
||
Logo Car Roth.gif
[http://www.carlroth.com/website/en-fr/carl-roth_index.jspp Carl Roth] |
 "
"