Team:Osaka/Tests
From 2011.igem.org
(→Tests) |
|||
| (71 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Osaka}} | {{Osaka}} | ||
__NOTOC__ | __NOTOC__ | ||
| + | <div class="padding"> | ||
| + | == Tests == | ||
| + | === Damage tolerance assay === | ||
| + | To measure the DNA damage tolerance conferred by each part, we used UV irradiation as a source of DNA damage and then assayed the survival rates. Transformed ''E. coli'' cells were plated on agar plates at different dilutions, air dried, and then exposed to different doses of UV radiation. Plates were wrapped with aluminum foil and incubated in the dark. Colony-forming units were scored after 16h incubation at 37°C. For detailed protocols, refer to the [https://2011.igem.org/Team:Osaka/Protocols Protocols page]. | ||
| - | == | + | The tolerance parts tested were as follows: |
| - | === | + | =====Parts containing one gene each===== |
| - | + | [[File:2011_osaka_tolerance_1.png|166px]] | |
| - | + | *CDS: PprI, PprA, PprM or RecA | |
| - | + | =====Parts containing two genes===== | |
| + | [[File:2011_osaka_tolerance_2.png|400px]] | ||
| + | *CDS1+2: PprI+RecA, PprA+RecA, PprM+RecA, PprI+PprA, PprI+PprM, PprA+PprM | ||
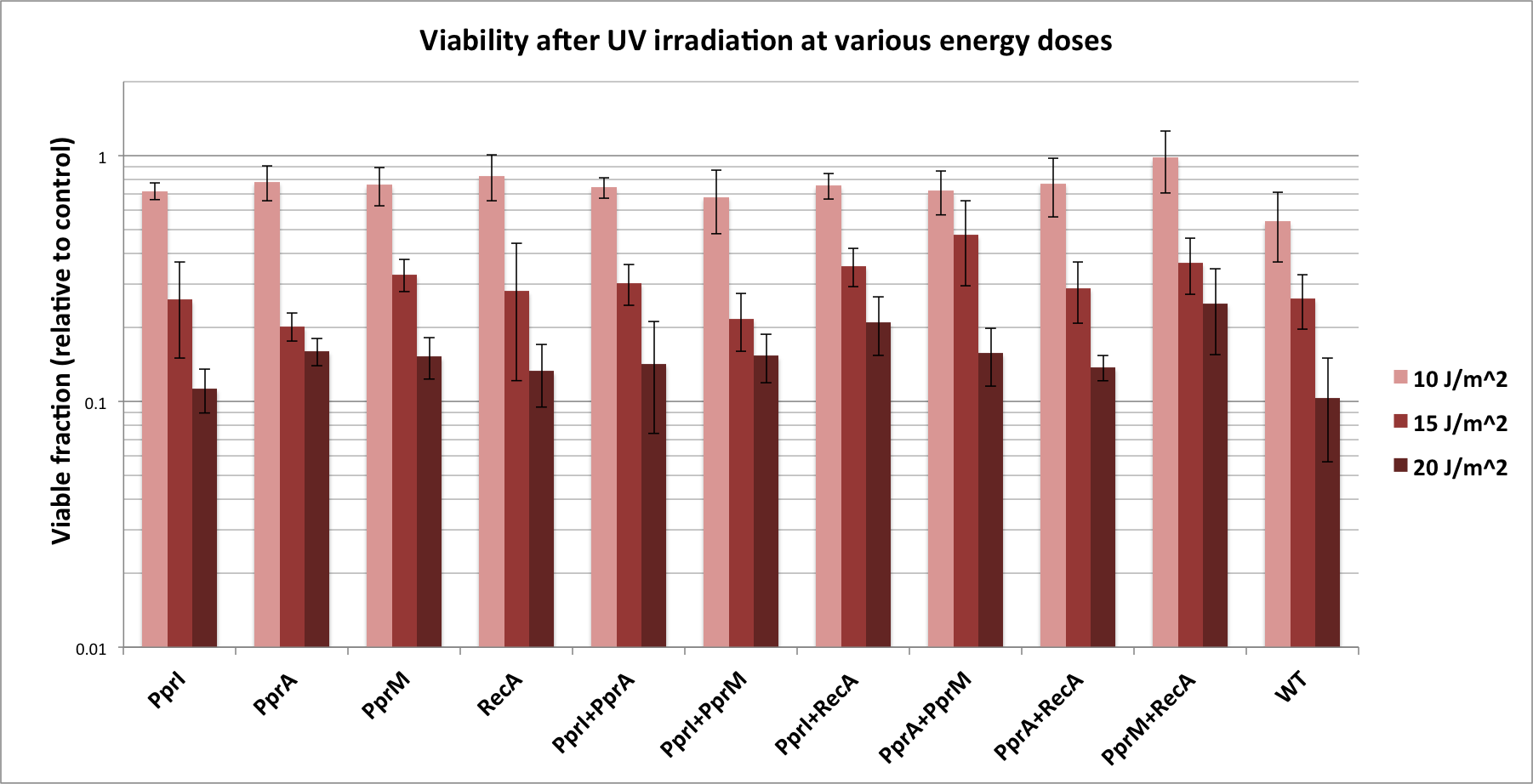
| - | + | Previously we tested some of these parts without IPTG induction. With the rationale that IPTG induction may increase expression of the protective proteins, we repeated past characterization tests with the inclusion of IPTG addition, and obtained slightly different results. These are indicated in the chart below. | |
| - | + | ||
| - | + | [[File:2011_osaka_tolerance_results.png|800px]] | |
| - | + | ||
| - | + | ==== Discussion ==== | |
| - | + | ===== Single-gene parts ===== | |
| - | + | *As before, PprI did not appear to significantly increase tolerance, corroborating with its known role as an inducer of other radiotolerance proteins. As ''E. coli'' would lack these required ''D. radiodurans'' proteins it is expected that PprI would not be able to confer tolerance on its own. | |
| + | *On the other hand, PprA, once induced with IPTG, seemed to confer a degree of DNA damage tolerance to ''E. coli''. This is not surprising considering PprA's function as a direct executor of DNA damage repair. | ||
| + | *''D. radiodurans'' RecA was indicated in our previous experimental results to confer the highest tolerance among the four radiotolerance genes; however, here, its effect was less than that of either PprA or PprM. This might be because it plays a complementary role to native ''E. coli'' RecA, thus the higher expression level induced by IPTG was of no additional benefit. | ||
| + | *Perhaps the most unexpected result was that from PprM. In our previous experiment (using non-induced parts), PprM also appeared to confer a degree of tolerance although it was not significant enough to be of note. Here, IPTG-induced PprM levels seem to provide a significant amount of DNA damage tolerance. This is somewhat in contradiction to literature which describes PprM as a modulator of the PprI-dependent damage response that depends on downstream effector proteins (PprA etc) to carry out its protective role. | ||
| - | + | ===== Two-gene combinations ===== | |
| - | + | *While PprI alone did not confer any tolerance, the combination of PprI and PprA worked to some degree. This is in accordance with the role of PprI as an inducer of PprA. What was interesting is that, PprA alone appeared to confer even higher tolerance. Perhaps expression of PprI is somehow detrimental to the host ''E. coli'' cells. | |
| + | *The combination of PprI and RecA produced high level of tolerance, which agrees with the role of PprI as an inducer of ''D. radiodurans'' RecA function. | ||
| + | *On the other hand, while literature mentions that PprM is not a modulator of RecA, here we see a significant increase in tolerance when these two genes are coupled. It could be explained by the fact that PprM is known to induce/modulate other, unknown proteins and some of these proteins may have homologs in ''E. coli'' that benefit from the presence of PprM. | ||
| + | ===== Conclusion ===== | ||
| + | We have obtained several interesting results from our DNA damage tolerance assays. First and foremost, PprM appears to confer a significant degree of tolerance to ''E. coli'', both on its own and in combination with other genes. Perhaps its role as a mere modulator of the PprI-dependent DNA damage response needs to be revised, or perhaps it is capable of regulating certain ''E. coli'' genes to advantageous effect. | ||
| + | In addition, our results indicated that PprI, as a global regulator of the DNA repair system, does not confer tolerance to ''E. coli''. This is in contradiction to a previous report that PprI confers radiotolerance to ''E. coli'' cells. Perhaps codon optimization and a better expression system are needed to make our PprI BioBrick functional. | ||
| - | + | Finally, we have shown that PprA, when expressed in sufficient quantities, does appear to confer tolerance to ''E. coli''. | |
| - | + | ||
| - | === | + | |
| - | + | === SOS promoter assay === | |
| - | < | + | <p>We assayed the promoter of the SOS gene RecA ([http://partsregistry.org/wiki/index.php?title=Part:BBa_J22106 J22106]), by attaching a lycopene biosynthesis gene cluster ([http://partsregistry.org/Part:BBa_K274100 K274100]) downstream as a reporter to yield the DNA damage detection device ([http://partsregistry.org/Part:BBa_K602013 K602013]). |
| - | + | Transformed ''E. coli'' was exposed to UV light and then incubated for 2 hours. Lycopene as a reporter was extracted from cells with acetone. For details check the [https://2011.igem.org/Team:Osaka/Protocols Protocols page].</p> | |
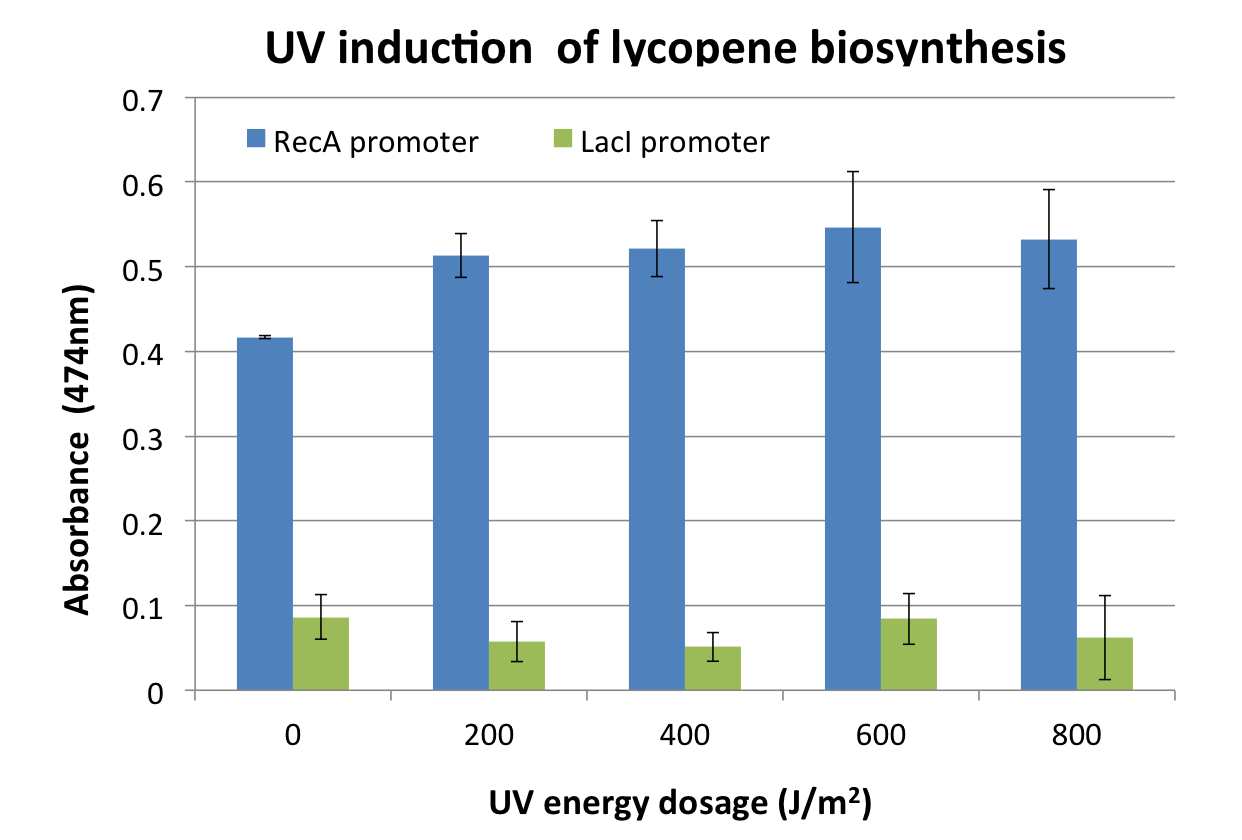
| + | <p>[[File:2011_osaka_promoter_1.png|400px]] | ||
| + | [[File:2011_osaka_promoter_2.png|400px]]</p> | ||
| + | |||
| + | <p>Response was defined as absorbance at 474nm (peak absorbance for lycopene) divided by OD600, followed by subtraction of background (non-irradiated samples) absorbance values. | ||
| + | We observed a response to UV irradiation that increased with energy dosage from 200 to 600 J/m^2. Response was decreased at 800 J/m^2, perhaps as a result of intensive DNA damage rendering lycopene biosynthesis genes non-functional.</p> | ||
| + | |||
| + | |||
| + | |||
| + | === Work in Progress === | ||
| + | ==== DNA damage tolerance ==== | ||
| + | *We are working on assembling a device with all four tolerance genes (PprI, PprA, PprM, RecA) but will not have time to characterize it properly before the wiki freeze. Stay tuned for our poster/presentation at the iGEM World Championship Jamboree for the results! | ||
| + | *To more properly measure the tolerance conferred by each part against DNA ''double strand breaks'' (the primary effect of ionizing radiation), we are working on characterizing the parts' tolerances against the drug Mitomycin C. Again, results will be too late for the wiki freeze so catch our presentation at the World Championship Jamboree! | ||
| + | |||
| + | ==== Damage detection ==== | ||
| + | * We have assembled a device utilizing GFP as reporter, but did not have time to characterize it properly. The results will be in our final presentation. | ||
Latest revision as of 21:22, 28 October 2011
Tests
Damage tolerance assay
To measure the DNA damage tolerance conferred by each part, we used UV irradiation as a source of DNA damage and then assayed the survival rates. Transformed E. coli cells were plated on agar plates at different dilutions, air dried, and then exposed to different doses of UV radiation. Plates were wrapped with aluminum foil and incubated in the dark. Colony-forming units were scored after 16h incubation at 37°C. For detailed protocols, refer to the Protocols page.
The tolerance parts tested were as follows:
Parts containing one gene each
- CDS: PprI, PprA, PprM or RecA
Parts containing two genes
- CDS1+2: PprI+RecA, PprA+RecA, PprM+RecA, PprI+PprA, PprI+PprM, PprA+PprM
Previously we tested some of these parts without IPTG induction. With the rationale that IPTG induction may increase expression of the protective proteins, we repeated past characterization tests with the inclusion of IPTG addition, and obtained slightly different results. These are indicated in the chart below.
Discussion
Single-gene parts
- As before, PprI did not appear to significantly increase tolerance, corroborating with its known role as an inducer of other radiotolerance proteins. As E. coli would lack these required D. radiodurans proteins it is expected that PprI would not be able to confer tolerance on its own.
- On the other hand, PprA, once induced with IPTG, seemed to confer a degree of DNA damage tolerance to E. coli. This is not surprising considering PprA's function as a direct executor of DNA damage repair.
- D. radiodurans RecA was indicated in our previous experimental results to confer the highest tolerance among the four radiotolerance genes; however, here, its effect was less than that of either PprA or PprM. This might be because it plays a complementary role to native E. coli RecA, thus the higher expression level induced by IPTG was of no additional benefit.
- Perhaps the most unexpected result was that from PprM. In our previous experiment (using non-induced parts), PprM also appeared to confer a degree of tolerance although it was not significant enough to be of note. Here, IPTG-induced PprM levels seem to provide a significant amount of DNA damage tolerance. This is somewhat in contradiction to literature which describes PprM as a modulator of the PprI-dependent damage response that depends on downstream effector proteins (PprA etc) to carry out its protective role.
Two-gene combinations
- While PprI alone did not confer any tolerance, the combination of PprI and PprA worked to some degree. This is in accordance with the role of PprI as an inducer of PprA. What was interesting is that, PprA alone appeared to confer even higher tolerance. Perhaps expression of PprI is somehow detrimental to the host E. coli cells.
- The combination of PprI and RecA produced high level of tolerance, which agrees with the role of PprI as an inducer of D. radiodurans RecA function.
- On the other hand, while literature mentions that PprM is not a modulator of RecA, here we see a significant increase in tolerance when these two genes are coupled. It could be explained by the fact that PprM is known to induce/modulate other, unknown proteins and some of these proteins may have homologs in E. coli that benefit from the presence of PprM.
Conclusion
We have obtained several interesting results from our DNA damage tolerance assays. First and foremost, PprM appears to confer a significant degree of tolerance to E. coli, both on its own and in combination with other genes. Perhaps its role as a mere modulator of the PprI-dependent DNA damage response needs to be revised, or perhaps it is capable of regulating certain E. coli genes to advantageous effect.
In addition, our results indicated that PprI, as a global regulator of the DNA repair system, does not confer tolerance to E. coli. This is in contradiction to a previous report that PprI confers radiotolerance to E. coli cells. Perhaps codon optimization and a better expression system are needed to make our PprI BioBrick functional.
Finally, we have shown that PprA, when expressed in sufficient quantities, does appear to confer tolerance to E. coli.
SOS promoter assay
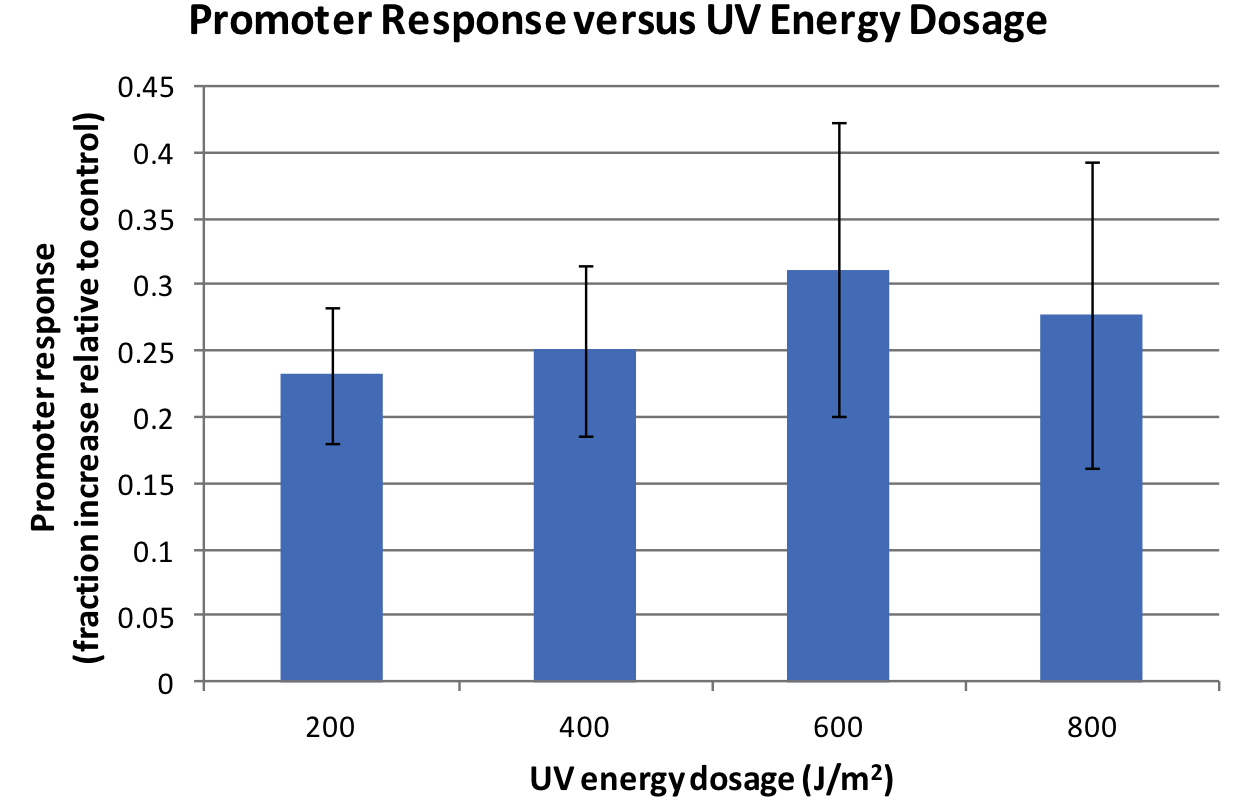
We assayed the promoter of the SOS gene RecA ([http://partsregistry.org/wiki/index.php?title=Part:BBa_J22106 J22106]), by attaching a lycopene biosynthesis gene cluster ([http://partsregistry.org/Part:BBa_K274100 K274100]) downstream as a reporter to yield the DNA damage detection device ([http://partsregistry.org/Part:BBa_K602013 K602013]). Transformed E. coli was exposed to UV light and then incubated for 2 hours. Lycopene as a reporter was extracted from cells with acetone. For details check the Protocols page.
Response was defined as absorbance at 474nm (peak absorbance for lycopene) divided by OD600, followed by subtraction of background (non-irradiated samples) absorbance values. We observed a response to UV irradiation that increased with energy dosage from 200 to 600 J/m^2. Response was decreased at 800 J/m^2, perhaps as a result of intensive DNA damage rendering lycopene biosynthesis genes non-functional.
Work in Progress
DNA damage tolerance
- We are working on assembling a device with all four tolerance genes (PprI, PprA, PprM, RecA) but will not have time to characterize it properly before the wiki freeze. Stay tuned for our poster/presentation at the iGEM World Championship Jamboree for the results!
- To more properly measure the tolerance conferred by each part against DNA double strand breaks (the primary effect of ionizing radiation), we are working on characterizing the parts' tolerances against the drug Mitomycin C. Again, results will be too late for the wiki freeze so catch our presentation at the World Championship Jamboree!
Damage detection
- We have assembled a device utilizing GFP as reporter, but did not have time to characterize it properly. The results will be in our final presentation.
 "
"