Team:NYMU-Taipei/immunological-solution
From 2011.igem.org
(→Our Two Constructs & Kill Switch) |
(→Our Two Constructs & Kill Switch) |
||
| Line 62: | Line 62: | ||
<font size=3>Delivering bacteria into human body may seem insane. But knowing that the total number of microbes that colonize the surface of our bodies is ten times greater than the total number of human, cell makes the whole thing less horrifying. The largest collection of symbiotic microbes reside in our gut, harbors trillions of bacteria, representing hundreds of species. However, scientists have not yet found any symbiotic bacteria residing in the human brain that does not cause infections. This year, the NYMU-Taipei team is thinking about enabling our magnetotactic bacteria being engulfed by a human glial cell in advance before being injected in to human brain.</font> | <font size=3>Delivering bacteria into human body may seem insane. But knowing that the total number of microbes that colonize the surface of our bodies is ten times greater than the total number of human, cell makes the whole thing less horrifying. The largest collection of symbiotic microbes reside in our gut, harbors trillions of bacteria, representing hundreds of species. However, scientists have not yet found any symbiotic bacteria residing in the human brain that does not cause infections. This year, the NYMU-Taipei team is thinking about enabling our magnetotactic bacteria being engulfed by a human glial cell in advance before being injected in to human brain.</font> | ||
| - | ==<font size=5><font color=crimson>'''Our Two Constructs | + | ==<font size=5><font color=crimson>'''Our Two Constructs'''</font></font>== |
<font size=4><font color=green>'''(1) MinC Construct'''</font></font> | <font size=4><font color=green>'''(1) MinC Construct'''</font></font> | ||
| Line 96: | Line 96: | ||
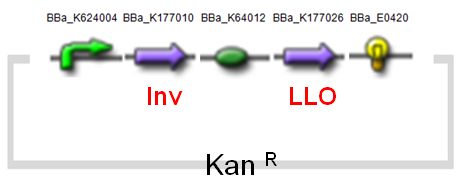
[[Image: AMB-1_Inv_LLO.jpg|center|frame|<center>Fig.7 Design of <i>inv.</i> & <i>LLO</i> Construct on AMB-1</center><center>Backbone Plasmid: pYMB</center>]]</font> | [[Image: AMB-1_Inv_LLO.jpg|center|frame|<center>Fig.7 Design of <i>inv.</i> & <i>LLO</i> Construct on AMB-1</center><center>Backbone Plasmid: pYMB</center>]]</font> | ||
| - | |||
| - | |||
==<font size=5><font color=crimson>'''Reference'''</font></font>== | ==<font size=5><font color=crimson>'''Reference'''</font></font>== | ||
Latest revision as of 23:22, 28 October 2011

Contents |
Background
For delivering the magnetotactic bacteria into the human brain and inducing an optogenetic response being our general concept, we, the NYMU-Taipei 2011 team, have tried hard focusing on eliminating the immune responses in brain at the same time.
Brain Inflammation
The brain is considered an immune privileged organ in that (i) it is separated from the rest of the body by a blood-brain-barrier, (ii) the relative absence of MHC class I and II expression on central nervous system (CNS) cells, and (iii) lack of abundant antigen presenting cells which are required for the generation of an adaptive immune response.
Hence, we suppose that the brain provides a pleasant, nurturing environment for parasites (bacteria in our case) due to structures that prevent many of the immunocytes from entering.
However, in spite of these facts, activated immunocytes such as T and B lymphocytes, monocytes, macrophages, and complement proteins readily accumulate in our brain once it is infected or injuried.
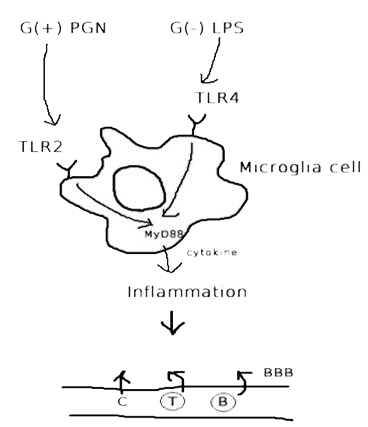
Microglial cell, tissue macrophages of brain, express most common macrophage markers provides the first line of defense in response to pathogens and neuronal injury. As such they can produce a wide variety of cytokines, proteases, etc….
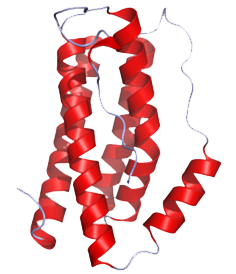
Cytokines - the key immune regulatory factors -, regulate communication between immune cells. There are cytokines such as tumor necrosis factor α (TNF), transforming growth factor β (TGF-β), interleukin 1 (IL-1), IL-6, IL-10 and IL-12,etc... Among these cytokines, our team has performed ELISA to detect the concentration of IL-6 (Fig.1), cytokine secreted by microglia cells when the brain tissue is infected or damaged to stimulate downstream immune responses and promote inflammation(Fig.2), and to confirm that our construct and design does decrease immune responses in brain.
| Fig.1 Structure of IL-6 (From: http://www.pdb.org/pdb/explore/explore.do?structureId=1ALU) | Fig.2 The process of immune responses triggered when being infected. |
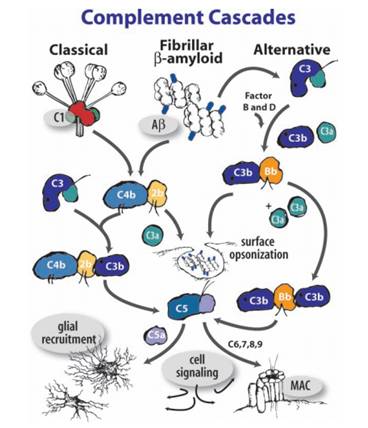
The complement system, another core component of the innate immune response, is consisted of small proteins found in blood. It has been confirmed that it can be activated and that it induces subsequence inflammation in the CNS. When being stimulated, proteases in the system cleave specific proteins to release cytokines, creating an amplifying cascade of further cleavages that result in the formation of membrane-attack-complex and ultimately pathogens elimination.(Fig.3)
Additionally, phagocytic cells including macrophages and neutrophils, can engulf and kill our bacteria.
Proposals on an "AMB-Itious" Solution
Magnetospirillum magneticum strain AMB-1 is an aquatic, motile, Gram-negative bacterium that usually resides in fresh water columns. It has no known pathogenic activity and is well regarded by many scientists for its safety. Still, we’d regarded that there are several core issues associated with what we have mentioned above.
To deal with problems, we have come up with several strategies to decrease and avoid immune responses.The ideas are (i) to express capsular polysaccharides of Klebsiella pneumonia and, (ii) being shielded in liposome.
(i) Klebsiella pneumonia is a Gram-negative opportunistic pathogen and a common cause of nosocomial infections. It possesses a polysaccharide capsule that is considered to be a major pathogenicity factor and the main contributing factor to its evasion ability from macrophages. However, expressing the capsule of K. pneumonia is not something easy. There are 77 different capsular antigens on the capsule, and certain serotypes are associated with certain infection sites. This would be a relatively huge challenge for us.
(ii) Liposomes have been wildly studied and suggested as a vehicle for drug delivery system, and it seems that this biocompatible, biodegradable lipid bilayer is our good choice. Still, being restricted to the short life time and the cost, we chose not to continue on the path.
The ideas above hold both advantages and drawbacks. After careful consideration, we have decided to utilize the system the Warsaw 2010 iGEM team project developed – BactoDHL, an invasive delivery system based on the expression of minC, Inv, and LLO. We redesigned and modified this system to be compatible with AMB-1 and in vitro symbiosis within glial cell.
Delivering bacteria into human body may seem insane. But knowing that the total number of microbes that colonize the surface of our bodies is ten times greater than the total number of human, cell makes the whole thing less horrifying. The largest collection of symbiotic microbes reside in our gut, harbors trillions of bacteria, representing hundreds of species. However, scientists have not yet found any symbiotic bacteria residing in the human brain that does not cause infections. This year, the NYMU-Taipei team is thinking about enabling our magnetotactic bacteria being engulfed by a human glial cell in advance before being injected in to human brain.
Our Two Constructs
(1) MinC Construct
In E. coli, the min system cooperates with the nucleoid to direct the placement of the division septum to midcell. MinC([http://partsregistry.org/wiki/index.php?title=Part:BBa_K624033 BBa_K624033]), the division inhibitor, is one of the gene products of the min system. Studies show that minC interacts directly with FtsZ and antagonizes FtsZ assembly. While overexpression of minC would lead to septation inhibition at all potential division sites, a circumstance of filamentation.
In our experiments, We have cloned the minC gene from E. coli K-12 strain MG1655 by performing PCR, with a single nucleotide synonymous mutation to eliminate the PstI enzyme cutting site.
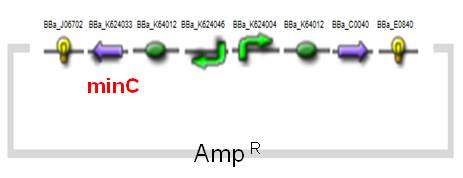
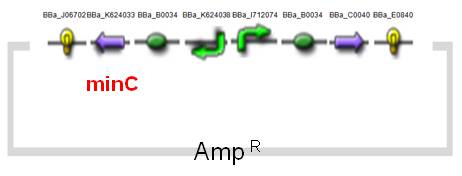
The expression of MinC is regulated by tetracycline-regulated system, which has been entirely researched and applied on AMB-1.The original design of a tetracycline-regulated gene expression system is based on two plasmids, one of which constitutively expresses a tetracycline-controlled transactivator protein (tTA), a fusion protein between the tetracycline repressor of E. coli and the transcriptional activation domain of the VP16 protein of herpes simplex virus. The second plasmid contains the gene to be regulated by tTA under the control of an inducible promoter which consists of seven copies of the tetracycline resistance operator (tetO). In our construct ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K624054 BBa_K624054], fig.4), we combined the two plasmids into one and reversed the minC sequence instead of back-inserting, in order to avoid the leakiness which may happen. See the construct applied on E. coli ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K624053 BBa_K624053], Fig.5).(See for Results.)
| Fig.4 Design of minC Construct on AMB-1 Backbone Plasmid: pYMB | Fig.5 Design of minC Construct on E.Coli Backbone Plasmid: pUC19 |
(2) Invasin & Listeriolysin O Constructs
An essential virulence attribute for Yersinia enterocolitica is its ability to invade the mammalian cell. Invasin, a protein encoded by inv, is an outer membrane protein found on the surface of Y. enterocolitica that is responsible for its binding property.
Listeriolysin O (LLO) (Fig.6) is a hemolysin produced by the bacterium Listeria monocytogenes, a pathogen that is responsible for listeriosis. LLO is activated within phagosomes of cells that have phagocytosed L. monocytogenes cells, and lyses the membrane of phagosome. The bacterium is then able to escape into the cytosol without damaging the plasma membrane, and grow intracellularly. This would result in the protection from extracellular immune system.
Below (Fig.7) is our modified design of AMB-1 (modified from 2010 Warsaw iGEM team's design).
Reference
- Huaijin Zhou and Joe Lutkenhaus (2005), MinC Mutants Deficient in MinD- and DicB-Mediated Cell Division Inhibition Due to Loss of Interaction with MinD, DicB, or a Septal Component J. Bacteriol., Apr 2005; 187: 2846 - 2857.
- Agha-Mohammadi, S., O'Malley, M., Etemad, A., Wang, Z., Xiao, X. and Lotze, M. T. (2004), Second-generation tetracycline-regulatable promoter: repositioned tet operator elements optimize transactivator synergy while shorter minimal promoter offers tight basal leakiness. The Journal of Gene Medicine, 6: 817–828
- J C Pepe and V L Miller (1990), The Yersinia enterocolitica inv gene product is an outer membrane protein that shares epitopes with Yersinia pseudotuberculosis invasin. J. Bacteriol., Jul 1990; 172: 3780 - 3789.
- Lane, Th.E.; Carson, M.; Bergmann, C.; Wyss-Coray, T. (Eds.) (2008), VIII, Springer Central Nervous System Diseases and Inflammation.
- Yu-Kuo Tsai, Chang-Phone Fung, Jung-Chung Lin, Jiun-Han Chen, Feng-Yee Chang, Te-Li Chen, and L. Kristopher Siu Klebsiella pneumoniae (2011), Outer Membrane Porins OmpK35 and OmpK36 Play Roles in both Antimicrobial Resistance and Virulence Antimicrob. Agents Chemother., Apr 2011; 55: 1485 - 1493.
- Fung CP, Hu BS, Chang FY, et al. (2000), A 5-year study of the seroepidemiology of Klebsiella pneumoniae: high prevalence of capsular serotype K1 in Taiwan and implication for vaccine efficacy. J Infect Dis 2000;181:2075–9
- Xu J, Mahowald MA, Ley RE, Lozupone CA, Hamady M, et al. (2007), Evolution of Symbiotic Bacteria in the Distal Human Intestine . PLoS Biol 5(7): e156. doi:10.1371/journal.pbio.0050156
- Gallia GL, Khalili K. (1998), Evaluation of an autoregulatory tetracycline regulated system. Oncogene. 1998;16:1879–1884.
- Pamela Schnupf, Daniel A. Portnoy, Listeriolysin O: a phagosome-specific lysin. Microbes and Infection 9 (2007) 1176e1187
 "
"