Team:NYC Wetware/Deinococcus/Data
From 2011.igem.org
(Difference between revisions)
| Line 20: | Line 20: | ||
<h3>Data For Our Favorite New Parts</h3> | <h3>Data For Our Favorite New Parts</h3> | ||
| - | <ol><a href="http://partsregistry.org/Part:BBa_K650007 Main Page"> main page</a> - <b>ruvA (E. coli), BBa_K650007</b>: | + | <ol>1. <a href="http://partsregistry.org/Part:BBa_K650007 Main Page"> main page</a> - <b>ruvA (E. coli), BBa_K650007</b>: RuvABC is a complex of three proteins that mediate branch migration and resolve the Holliday junction created during homologous recombination in bacteria. As such, RuvABC is critical to bacterial DNA repair. RuvA and RuvB bind to the four strand DNA structure formed in the Holliday junction intermediate, and migrate the strands through each other, using a putative spooling mechanism.<br/> |
| - | <a href="http://partsregistry.org/Part:BBa_K650008 Main Page"> main page</a> - <b>ruvB (E. coli), BBa_K650008</b>: May play a role in the repair of endogenous alkylation damage. | + | <br/> |
| - | <a href="http://partsregistry.org/Part:BBa_K650009 Main Page"> main page</a> - <b>radA (E. coli), BBa_K650009</b>: May play a role in the repair of endogenous alkylation damage. RadA takes part in conjugational recombination and DNA damage repair. Belongs to the RecA family.<br/> | + | 2. <a href="http://partsregistry.org/Part:BBa_K650008 Main Page"> main page</a> - <b>ruvB (E. coli), BBa_K650008</b>: May play a role in the repair of endogenous alkylation damage. RuvABC is a complex of three proteins that mediate branch migration and resolve the Holliday junction created during homologous recombination in bacteria. As such, RuvABC is critical to bacterial DNA repair. RuvA and RuvB bind to the four strand DNA structure formed in the Holliday junction intermediate, and migrate the strands through each other, using a putative spooling mechanism.<br/> |
| - | <a href="http://partsregistry.org/Part:BBa_K650010 Main Page"> main page</a> - <b> | + | <br/> |
| - | <a href="http://partsregistry.org/Part:BBa_K650011 Main Page"> main page</a> - <b>Tpx (E. coli), BBa_K650011</b>: Oxidative stress defenses combat reactive oxygen species such as superoxide (O), hydrogen peroxide (H2O2) and the hydroxyl radical (OH) generated by the host immune response, environmental factors, and the incomplete reduction of oxygen to water during aerobic respiration, all of which are hazardous to proteins, DNA, and lipids. E. coli Tpx (thiol peroxidase) is part of an oxidative stress defense system that uses reducing equivalents from thioredoxin (Trx1) and thioredoxin reductase to reduce alkyl hydroperoxides. | + | 3. <a href="http://partsregistry.org/Part:BBa_K650009 Main Page"> main page</a> - <b>radA (E. coli), BBa_K650009</b>: May play a role in the repair of endogenous alkylation damage. RadA takes part in conjugational recombination and DNA damage repair. Belongs to the RecA family.<br/> |
| + | <br/> | ||
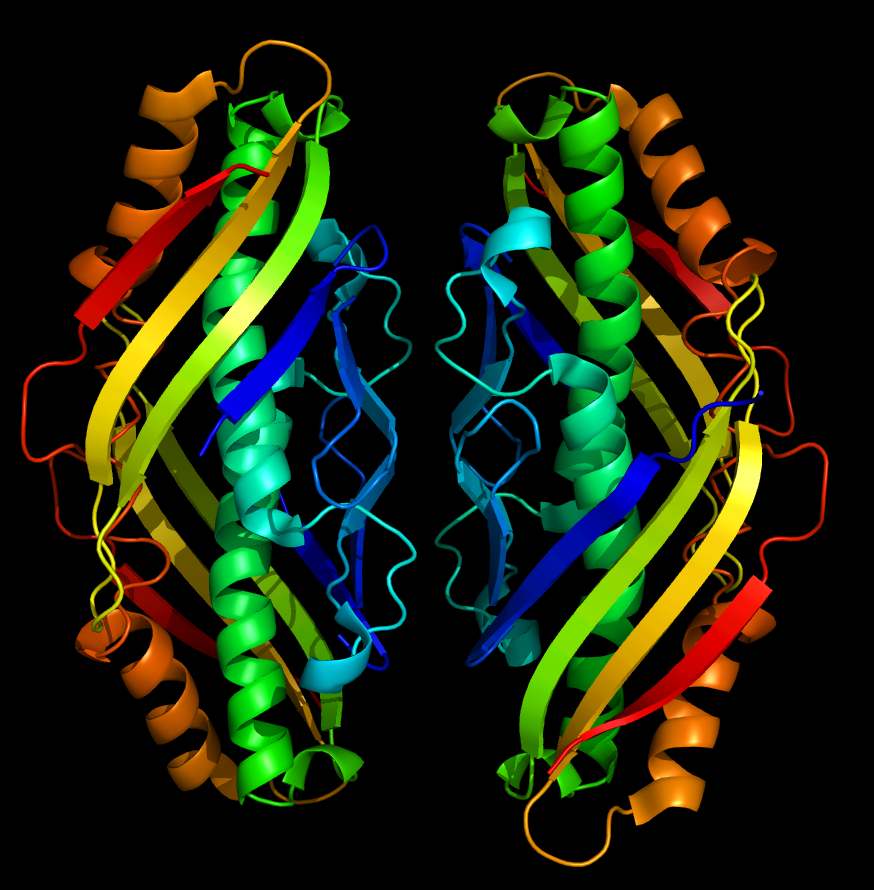
| + | 4. <a href="http://partsregistry.org/Part:BBa_K650010 Main Page"> main page</a> - <b>osmC (E. coli), BBa_K650010</b>: Osmotically inducible, stress-inducible membrane protein; involved in defense against oxidative compounds; required for long-term survival in stationary phase. Fold a beautiful protein <img src="http://4.bp.blogspot.com/_HK1LuizCXdI/TA6_LG2XgMI/AAAAAAAABh0/sjlzNC_5LiU/s1600/1vla_Hydroperoxide_reistance_protein_osmc.png"><br/> | ||
| + | <br/> | ||
| + | 5. <a href="http://partsregistry.org/Part:BBa_K650011 Main Page"> main page</a> - <b>Tpx (E. coli), BBa_K650011</b>: Oxidative stress defenses combat reactive oxygen species such as superoxide (O), hydrogen peroxide (H2O2) and the hydroxyl radical (OH) generated by the host immune response, environmental factors, and the incomplete reduction of oxygen to water during aerobic respiration, all of which are hazardous to proteins, DNA, and lipids. E. coli Tpx (thiol peroxidase) is part of an oxidative stress defense system that uses reducing equivalents from thioredoxin (Trx1) and thioredoxin reductase to reduce alkyl hydroperoxides. | ||
<br/> | <br/> | ||
</ol> | </ol> | ||
Revision as of 06:28, 28 September 2011

Data For Our Favorite New Parts
- 1. main page - ruvA (E. coli), BBa_K650007: RuvABC is a complex of three proteins that mediate branch migration and resolve the Holliday junction created during homologous recombination in bacteria. As such, RuvABC is critical to bacterial DNA repair. RuvA and RuvB bind to the four strand DNA structure formed in the Holliday junction intermediate, and migrate the strands through each other, using a putative spooling mechanism.
2. main page - ruvB (E. coli), BBa_K650008: May play a role in the repair of endogenous alkylation damage. RuvABC is a complex of three proteins that mediate branch migration and resolve the Holliday junction created during homologous recombination in bacteria. As such, RuvABC is critical to bacterial DNA repair. RuvA and RuvB bind to the four strand DNA structure formed in the Holliday junction intermediate, and migrate the strands through each other, using a putative spooling mechanism.
3. main page - radA (E. coli), BBa_K650009: May play a role in the repair of endogenous alkylation damage. RadA takes part in conjugational recombination and DNA damage repair. Belongs to the RecA family.
4. main page - osmC (E. coli), BBa_K650010: Osmotically inducible, stress-inducible membrane protein; involved in defense against oxidative compounds; required for long-term survival in stationary phase. Fold a beautiful protein
5. main page - Tpx (E. coli), BBa_K650011: Oxidative stress defenses combat reactive oxygen species such as superoxide (O), hydrogen peroxide (H2O2) and the hydroxyl radical (OH) generated by the host immune response, environmental factors, and the incomplete reduction of oxygen to water during aerobic respiration, all of which are hazardous to proteins, DNA, and lipids. E. coli Tpx (thiol peroxidase) is part of an oxidative stress defense system that uses reducing equivalents from thioredoxin (Trx1) and thioredoxin reductase to reduce alkyl hydroperoxides.
 "
"