Team:Macquarie Australia/Project
From 2011.igem.org
(→Primer Design) |
(→Our project) |
||
| Line 3: | Line 3: | ||
<br> | <br> | ||
| - | =Our project= | + | ='''Our project'''= |
| - | ==Background== | + | =='''Background'''== |
| Line 20: | Line 20: | ||
The bacteriophytochrome vector will be transformed into E.coli competent BL21 (DE3) cells. However E.coli does not contain a hemeoxygenase gene which is why it must be incorporated into the genetically engineered operon along with a T7 promoter/terminator and the bacteriophytochrome genes from Deinococcus and Agrobacterium. <br> | The bacteriophytochrome vector will be transformed into E.coli competent BL21 (DE3) cells. However E.coli does not contain a hemeoxygenase gene which is why it must be incorporated into the genetically engineered operon along with a T7 promoter/terminator and the bacteriophytochrome genes from Deinococcus and Agrobacterium. <br> | ||
| - | ==Aims== | + | =='''Aims'''== |
The aims of the team this year are to complete the light switch construction: | The aims of the team this year are to complete the light switch construction: | ||
| Line 44: | Line 44: | ||
3. Assemble an operon consisting of the heme-oxygenase and bacteriophytochrome genes. | 3. Assemble an operon consisting of the heme-oxygenase and bacteriophytochrome genes. | ||
4. Optimise the gene expression from the operon such that the bacteriophytochrome light switch assembles without requiring the addition of biliverdin. | 4. Optimise the gene expression from the operon such that the bacteriophytochrome light switch assembles without requiring the addition of biliverdin. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
= '''The Experiments''' = | = '''The Experiments''' = | ||
Revision as of 14:37, 1 October 2011
Contents |
Our project
Background
Phytochromes were first discovered in plant and were found to be associated with the plants photoperception. The phytochrome proteins act as light sensors by absorbing red/far red light, with some their range can be between the UV-A to far-red regions. Their main function was found to mediate various time-dependant events such as seed germination, seedling de-etiolation and the generation of flowers.
Bacteriophytochromes also act as photoreceptors absorbing in the red to far-red light, they mediate sensory responses to their light environment, the alignment of their circadian system's can function as a quorum-sensing network. Bacteriophytochromes can act as protein kinases sensitive to the light which are able to modify or regulate various components in the cell, such as protein activity and the synthesis of the light harvesting complex via the promotion of transcription factors. They control genes associated with various rhythm-dependant proteins at the transcription level by regulation of transcription factors to the promoter regions depending on their binding affinity. By doing so, they create what is called, a “light switch”. Techniques can be used to temporarily inactivate specific proteins by using a photolabile protecting group; this is referred to as “caging”. The light switch bacteriophytochrome that is utilised by Agrobacterium and Deinococcus has a domain structure as follows, PAS-GAF-PHY-HisKinase and is able to cause a shift in conformation of the chromophore complex according to the wavelength absorbed.
For a light switch to be self-sufficient and fully functional, two main components are required, a hemeoxygenase and a bacteriophytochrome coupled with ribosome binding sites also known as the Shine-Dalgarno sequence. The hemeoxygenase is required for the degradation of the porphyrin heme to form the linear tetrapyrrole biliverdin which is then able to autocatalytically bind to the bacteriophytochrome on the GAF domain of the bacteriophytochrome.
This forms the chromophore complex producing a colour according to the ratio of red to far-red light absorbed. Various processes are influenced by phytochromes through photoconversion between two isomers, a phytochrome red biologically inactive form (Pr) that absorbs red light and a phytochrome far red active form which absorbs far-red light. Pr expresses a blue colour that is able to isomerise to a green colour when absorption of far-red light is greater.
The bacteriophytochrome vector will be transformed into E.coli competent BL21 (DE3) cells. However E.coli does not contain a hemeoxygenase gene which is why it must be incorporated into the genetically engineered operon along with a T7 promoter/terminator and the bacteriophytochrome genes from Deinococcus and Agrobacterium.
Aims
The aims of the team this year are to complete the light switch construction:
1. Remove the restriction sites from the bacteriophytochrome and heme-oxygenase genes which are incompatible with biobrick assembly.
2. Assemble a fully functional bacteriophytochrome biobrick which is functionally expressed in E.coli.
3. Assemble an operon consisting of the heme-oxygenase and bacteriophytochrome genes.
4. Optimise the gene expression from the operon such that the bacteriophytochrome light switch assembles without requiring the addition of biliverdin.
== Introducing a functional light switch into E. coli.
==
The objective in this project is to build and characterise a biological light switch in E.coli. This will involve construction of bacteriophytochrome biobrick parts and heme-oxygenase biobrick parts. In 2010 the Macquarie Team cloned bacteriophytochrome from two sources and showed that one was functionally assembled when incubated with biliverdin. The part created is not directly as a biobrick as it contains an internal PstI site and the XbaI biobrick site is missing. The heme-oxygenase clone also contains an internal restriction site which is not compatible with biobrick assembly. The aims of the team this year are to complete the light switch construction: 1. Remove the restriction sites from the bacteriophytochrome and heme-oxygenase genes which are incompatible with biobrick assembly. 2. Assemble a fully functional bacteriophytochrome biobrick which is functionally expressed in E.coli. 3. Assemble an operon consisting of the heme-oxygenase and bacteriophytochrome genes. 4. Optimise the gene expression from the operon such that the bacteriophytochrome light switch assembles without requiring the addition of biliverdin.
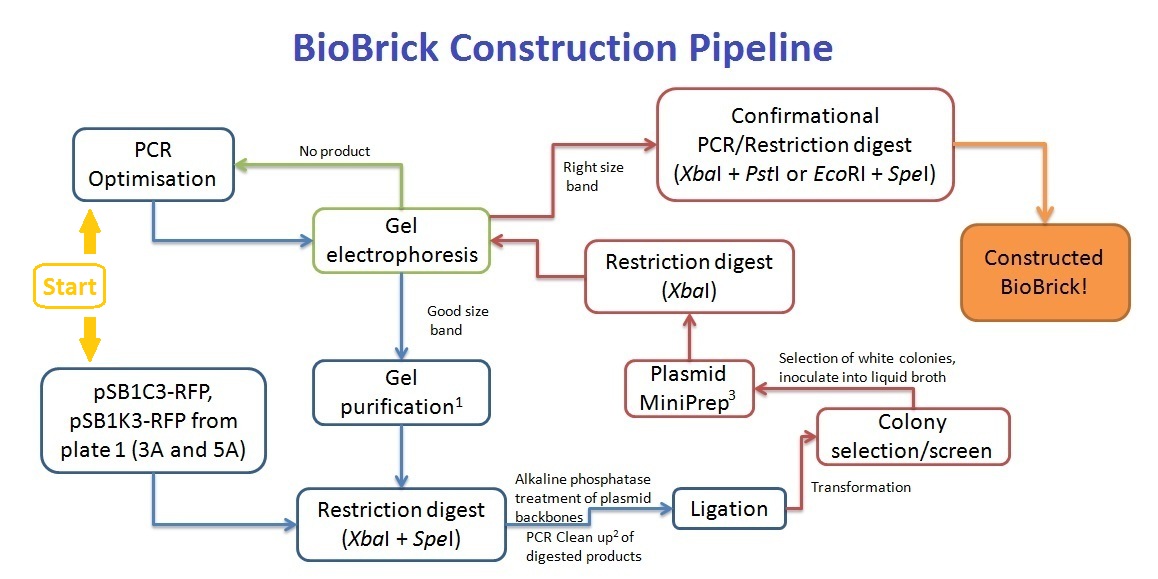
The Experiments
BioBrick Construction
1 - Qiagen Gel extraction kit, 2 - Sigma PCR clean up kit, 3 - Qiagen Spin Miniprep kit
Primer Design
Primer Sequence Melting temp (°C) BB-FWD 5'- GAA TTC GCG GCC GCT TCT AGA -3' 59.7 BB-REV 5'- CTG CAG CGG CCG CTA CTA GTA -3' 61 T7-BB-FWD 5'- GAA TTC GCG GCC GCT TCT AGA TCT CGA TCC CGC G -3' 69.4 T7-BB-REV 5'- CTG CAG CGG CCG CTA CTA GTA GAG GGG AAT TGT TAT -3' 66.5 HO1-BB-FWD 5'- GCG GCC GCT TCT AGA CTT TAA GAA GGA GAT ATA C -3' 61.9 HO1-BB-REV 5'- GCG GCC GCT ACT AGT ACT TTC GGG CTT TGT TAG C -3' 67 DR-BB-FWD 5'- TCG CGG CCG CTT CTA GAA GGA GGG CTG CTA TGA GC -3' 71 DR-BB-REV 5'- GCG GCC GCT ACT AGT ATC AGG CAT CGG CGG CTC CCG G -3' 68.7 DR-M-FWD 5'- TTG CTG ATT CTG GAG TTC GAG CCG ACG GAG -3' 63.5 DR-M-REV 5'- CTC CGT CGG CTC GAA CTC CAG AAT CAG CAA -3' 61.7 AT-BB-FWD 5'- CGC GGC CGC TTC TAG AGA TTA GGA GGG CTG CTA TGA G -3' 69.1 AT-BB-REV 5'- GCG GCC GCT ACT AGT AGA TTT CAG GCA ATT TTT TCC -3' 64.3 AT-M1-FWD 5'- GTC CCT GCA TTA TCT TCA GAT GAT ATC AGA -3' 53.8 AT-M1-REV 5'- TCT GAT ATC ATC TGA AGA TAA TGC AGG GAC -3' 55.7 AT-M2-FWD 5'- GTG TTT CAG AGG CTT CAG CGG GTG GAG GA -3' 63.9 AT-M2-REV 5'- TCC TCC ACC CGC TGA AGC CTC TGA AAC AC -3' 64.5
 "
"