Week 1: June 6 - June 11
In the first week we began constructing basic reporter DNA constructs that have various combinations of promoters and genes. The mix-and-matching of promoters and genes was done using the LR reaction following the Gateway protocol. Our promoters included TRE, minCMV, Hef1a, Hefl1a-LacO, and our genes included eYFP, mKate, and eBFP2. Transformed and inoculated colonies from the LR reactions and performed restriction digests. Approximately half of the LR reactions were successful and became DNA parts that we could use later on.
June 7, 2011
Date
|
Assignee
|
DEST_R4R2
|
L4-R1 Promoter
|
L1-L2
Gene
|
LR?
|
Tube Number
|
Colony Number,
Antibiotic, Person
|
Protocol Used |
6.7.2011
|
Michelle
Louis
|
2-3
|
Tre
|
C1434VP16
|
Yes. 6.7.2011, 5pm
|
11
|
5, AMP, KH
|
Standard, 30C for 16h
|
6.7.2011
|
Michelle
Louis
|
2-3
|
Tre
|
Mnt1VP16
|
Yes. 6.7.2011, 5pm
|
12
|
4, AMP, KH |
Standard, 30C for 16h |
6.7.2011
|
Tyler
|
2-3
|
Tre
|
LacIKrab
|
Yes. 6.7.2011, 5pm
|
6
|
30, AMP, KH |
Standard, 30C for 16h |
6.7.2011
|
Jenny
Divya
|
3-4
|
minCMV-7xMnt1
|
eYFP
|
Yes. 6.7.2011, 5pm
|
9
|
0, AMP, KH, needs to be redone
|
Standard, 30C for 16h |
6.7.2011
|
Jenny
Divya
|
2-3
|
Tre
|
LexAVP16
|
Yes. 6.7.2011, 5pm
|
10
|
2, AMP, KH, needs to be redone
|
Standard, 30C for 16h |
| 6.7.2011 |
Mariola
Kenneth
|
3-4
|
minCMV 1xC1434
|
eYFP
|
Yes. 6.7.2011, 5pm
|
7
|
3, AMP, KH, needs to be redone |
Standard, 30C for 16h |
| 6.7.2011 |
Mariola
Kenneth |
3-4 |
minCMV 4xLexA
|
eYFP
|
Yes. 6.7.2011, 5pm
|
8
|
0, AMP, KH, needs to be redone
|
Standard, 30C for 16h |
LR Reactions
Utilizing protocol here: LR Protocol
Date
|
Assignee
|
DEST_R4R2
|
L4-R1 Promoter
|
L1-L2
Gene
|
LR?
|
Tube Number
|
Colony Number,
Antibiotic, Person
|
Protocol Used |
6.9.2011
|
Grant
|
3-4
|
minCMV 4xLexA |
eYFP |
Yes. 6.9.2011, 3pm
|
12
|
10, AMP, GR
|
Standard + Grown at 37C in LB not SOC. |
6.9.2011
|
Jon C
|
3-4
|
minCMV-4xMnt1
|
eYFP
|
Yes. 6.9.2011, 3:45pm |
2
|
13, AMP, GR
|
Standard + Grown at 37C in LB not SOC. |
6.9.2011
|
Jon C
|
3-4
|
minCMV-4xCI434
|
eYFP
|
Yes. 6.9.2011, 3:45pm |
1
|
7, AMP, GR |
Standard + Grown at 37C in LB not SOC. |
6.9.2011
|
Jon C
|
3-4
|
Hef1a-LacO
|
eYFP
|
Yes, 6.9.2011, 4:20pm
|
5
|
25, AMP, GR
|
Standard + Grown at 37C in LB not SOC. |
6.9.2011
|
Semon
|
4-5
|
Tre
|
mKate
|
Yes, 6.9.2011 4:27pm
|
3
|
6, AMP, GR
|
Standard + Grown at 37C in LB not SOC. |
6.9.2011
|
Semon
|
1-2
|
Hef1a
|
eBFP2
|
Yes, 6.9.2011, 4.27pm
|
4
|
3, AMP, GR
|
Standard + Grown at 37C in LB not SOC. |
Transformation
Date
|
Assignee
|
DEST_R4R2
|
L4-R1 Promoter
|
L1-L2
Gene
|
LR?
|
|
Colony Number,
Antibiotic
|
Protocol Used |
6.9.2011
|
Mariola
|
|
|
eYFP
|
no. Replication stock. |
|
2, AMP
|
Transformation (~ 2 hr 20 min) using LB instead of SOC |
Miniprep
| Date |
Assignee
|
DNA
|
Quantity
|
Time collected
|
6.9.2011
|
Divya-Jenny
|
pEXPR_2-3_Tre:LexAVP16
|
52.2 ng/uL
|
12pm
|
6.9.2011
|
Kenneth
|
pEXPR_3-4_minCMV-CI434:eYFP (A)
|
70 ng/ul
|
12pm
|
6.9.2011
|
Kenneth |
pEXPR_3-4_minCMV-CI434:eYFP (B)
|
174 ng/uL
|
12pm
|
6.9.2011
|
Louis
|
pEXPR_2-3_Tre:C1434VP16
|
234.6 ng/uL
|
12pm
|
6.9.2011
|
Tyler
|
pEXPR_2-3_Tre:Lac/Krab
|
130.7 ng/uL
|
12pm
|
6.9.2011
|
Michelle
|
pEXPR_2-3_Tre:Mnt1VP16
|
110.0 ng/uL
|
12pm
|
6.9.2011
|
Michelle
|
pDEST_2-3_ccdB
|
117.6 ng/uL
|
12pm
|
Restriction Digests
Assignee
|
DNA
|
Enzyme
|
Expected Results
|
Picture of Gel
|
Time Incubated |
Comments
|
Louis
|
pEXPR_2-3_Tre:C1434VP16 |
NdeI
|
6700 bp
800 bp |
a.3 |
6/9/11
3:50 PM |
|
|
|
Kenneth
|
pEXPR_3-4_minCMV-CI434:eYFP (A+B)
|
NcoI and SacII
|
2450 bp
4650 bp
|
A: a.6
B: a.7
|
6/9/11
3:00 PM
|
A did not cut as expected. B looks good.
Digestion Mix: 2 uL NEB4, 0.5 uL of each enzyme, A: 7.1 uL/B: 2.9 uL of DNA, 10.4 uL/14.6 uL of H20
|
|
|
Michelle
|
pEXPR_2-3_Tre:Mnt1VP16
|
BglI
|
3700 bp
2200 bp
1300 bp
|
a.1 |
6/9/11
4:00PM
|
DNA appeared to be uncut. Need to redo, possibly with a new enzyme and more DNA.
|
|
|
Michelle
|
pDEST_2-3_ccdB
|
NcoI and NheI
|
6100 bp
1700 bp
|
a.2 |
6/9/2011
4:25 PM
|
Attained bands at correct positions. Other band represents partially cut DNA in double digest.
|
|
|
Divya
|
pEXPR_2-3_Tre:LexAVP16
|
HincII
|
|
a.4 |
6/9/2011
4:45 PM
|
- Bands didn't show up. Needs to be redone.
- DNA may have degraded. Need to redo Nanodrop as well. |
|
|
| Tyler |
pEXPR_2-3_Tre:Lac/Krab |
SalI |
3 bands:
5288 bp
1510 bp
1241 bp
cuts gene |
a.5 |
6/9/2011
3:00 PM |
Digestion:
4 uL * 130.7 ng/uL = 522.8 ng DNA
2 uL NEB3
2 uL BSA
11 uL H2O
1 uL SalI
Total: 20 uL
Only saw one band at 3500 bp
Needs to be redone possibly with another restriction enzyme |
|
|
Gels
| Label |
Picture |
| a |
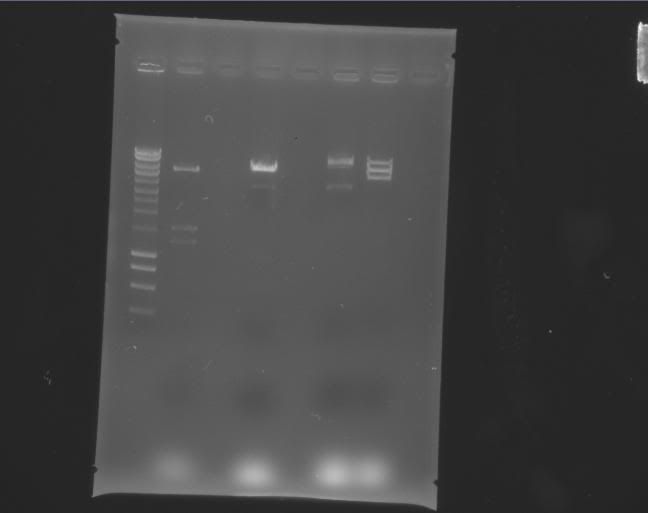
|
June 10, 2011
Restriction Gels
| Assignee |
DNA
|
Enzyme
|
Expected result
|
Time Incubated
|
Comments
|
Louis
|
pEXPR_2-3_Tre:C1434VP16 |
EcoRI (Buffer 2)
|
5500 bp
1050 bp
650 bp
360 bp |
6/10/11
11:15 AM |
|
Michelle
|
pEXPR_2-3_TRE:Mnt1VP16 (A)
|
NcoI and NheI
|
5700 bp
1300 bp
|
6/10/11
11:40 AM
|
Digestion:
6 uL DNA
1 uL NcoI
1 uL Nhe1
2 uL NEB2
2 uL BSA
8 uL H2O
Failed.
|
Michelle
|
pEXPR_2-3_TRE:Mnt1VP16 (B)
|
NheI and SphI
|
1700 bp
5300 bp
|
6/10/11
11:40 AM
|
Digestion:
6 uL DNA
1 uL NheI
1 uL SphI
2 uL NEB2
2 uL BSA
8 uL H2O
Failed. Redoing LR on Monday June 13th.
|
| Tyler |
pEXPR_2-3_Tre:Lac/Krab |
SalI |
3 bands:
5288 bp
1510 bp
1241 bp
cuts gene |
6/10/2011
3:00 PM |
Digestion: 4 uL * 130.7 ng/uL = 522.8 ng DNA
2 uL NEB3
2 uL BSA
11 uL H2O
1 uL SalI
Total: 20 uL
***Worked (see gel below - b.1)
Stored in -80C freezer |
Comments:
pEXPR_2-3_Tre:LexAVP16 and pEXPR_3-4_minCMV-7xMnt1:eYFP are being entirely redone.
pEXPR_2-3_Tre:LexAVP16 (post-miniprep) was lost. pEXPR_3-4_minCMV-7xMnt1:eYFP LR was unsuccessful (no bacteria grew).
LR and transformation will be done on Saturday. Miniprep and restriction mapping will be done on Sunday
| Label |
Gel
|
Legend
|
b
|
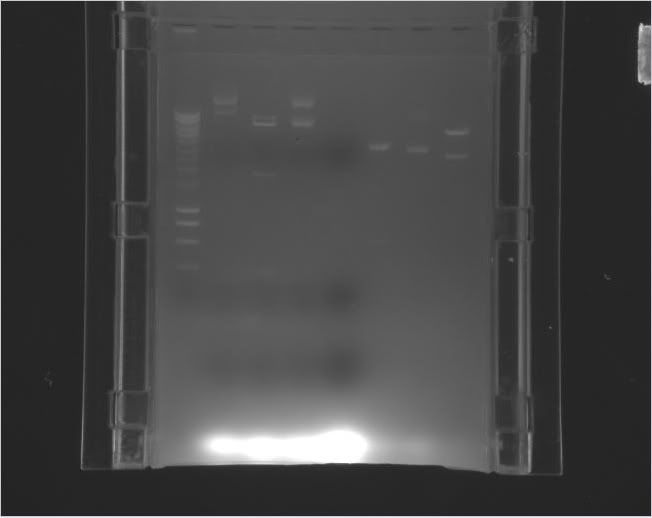
|
Column 0: HyperLadder
Column 1: pEXPR_2-3_Tre:Lac/Krab
Column 4: pEXPR_2-3_Tre:C1434VP16
Column 6: pEXPR_2-3_TRE:Mnt1VP16 (A)
Column 7: pEXPR_2-3_TRE:Mnt1VP16 (B) |
June 11, 2011
Set of LRs from 6/9 (GR, JC, and SR) cell counts taken; inoculated by GR/JC. 3 cells taken per plate.
Assignee
|
DNA
|
Enzyme
|
Expected Results
|
Time Incubated
|
Comments
|
Sam
|
pEXPR_1-2_Hef:eBFP
|
NcoI & ApaL1
|
3750bp, 3180bp, 1250bp |
45 min |
If it doesn't work there should be a whole mess of fragments (including a small 600bp one)
1mL each enz; 2 mL Buf; 1 mL BSA; 2mL DNA; 13mL H2O (Standard Protocol with extra BSA)
|
Sam
|
pEXPR_4-5_Tre:mKate
|
Bgl1
|
1270bp, 2630bp, 3380bp |
45 min |
1mL enz; 2mL DNA; 2mL BSA; 2mL Buf; 15mL H2O (Standard Protocol with extra BSA and water - not quite 10x)
|
Week 2: June 12 - June 18
In the second week we attempted midipreps instead of minipreps of our available cell stocks. Midipreps were unsuccessful, likely due to centrifuge limitations. LR reactions to generate more usable promoter-gene pair parts continued, expanding to include the CI434-VP16, LexA-VP16, and Mnt-VP16 genes, whose expressed proteins activate the appropriate corresponding minCMV promoters. Most of the LRs done were not successful, reason unknown at this time.
June 13, 2011
Status
| DNA |
Status
|
Comment
|
Mntcmv/1x cI434:eYFP
|
good
|
|
Mnt cmv/4x LexA:eYFP
|
bad |
|
HefIa/LacO:eYFP
|
good |
|
Tre:LacI Krab
|
good |
|
HefIaL EBFP2
|
bad |
|
Date
|
Assignee
|
DEST_R4R2
|
L4-R1 Promoter
|
L1-L2
Gene
|
LR?
|
Tube Number
|
Colony Number,
Antibiotic, Person
|
Protocol Used |
6.13.2011
|
Jon
|
2-3
|
Tre |
C1434-VP16 |
Yes. 6-13, 11am
|
10
|
about 50, AMP, JC
|
No |
6.13.2011
|
Tyler
|
2-3
|
Tre
|
LexA-VP16
|
Yes. 6-13, 11am |
9
|
about 30, AMP, GR
|
No |
6.13.2011
|
Mariola
|
2-3
|
Tre
|
Mnt1-VP16
|
Yes. 6-13, 11am |
Eppendorf
|
0, AMP, GR |
No |
6.13.2011
|
Semon
|
4-5
|
Tre
|
mKate
|
Yes. 6-13, 11am
|
11
|
about 10, AMP, SR
|
No |
6.13.2011
|
Divya
|
3-4
|
minCMV-7xMnt1
|
eYFP
|
Yes 6/13, 1:30pm
|
?
|
1000s, AMP, GR
|
No |
6.13.2011
|
Jenny
|
3-4
|
minCMV-4xMnt1
|
eYFP
|
Yes 6/13, 1:30pm
|
?
|
1000s, AMP, GR
|
No |
6.13.2011
|
Jenny
|
3-4
|
minCMV-4xC1434
|
eYFP
|
Yes 6/13,1:30pm
|
?
|
1000s, AMP, GR
|
No |
June 14, 2011
Highlights:
~10:30 AM: Miniprep of pDEST23-ccdB commenced and completed. DNA concentrations of the two samples were 175 and 310 ng/uL
~1:00 PM: Inoculation of cultures for pEXPR-23-TRE-CI434-VP16, pEXPR-23-TRE-LexA-VP16, pEXPR-45-TRE-mKate, pEXPR-34-minCMV-7xMnt-eYFP, pEXPR-34-minCMV-4xMnt-eYFP, and pEXPR-minCMV-4xCI434 were conducted. Colony counts from the previous transformations are noted above.
~1:30 PM: LR Gateway reactions were performed for the failed pEXPR-23-TRE-Mnt-VP16 and the new pEXPR-12-Hef1A-rtTA.
~5:00 PM: Inoculation of midiprep cultures for EXPR-34-minCMV4xLexA-eYFP, two samples of pEXPR-34-Hef1ALacO-eYFP, pEXPR-12-Hef1A-EBFP, pEXPR-23-TRE-LacIKrab, and pEXPR-34-minCMV1xcI434-eYFP commenced. We plan to midiprep the cells at about 10:00 AM.
~6:00 PM: Transformation of the aforementioned LR Gateway reactions commenced. Transformation completed around 9:00 PM.
~7:00 PM: Design for the TANGO primers complete.
June 15, 2011
~4AM: Grant did more LRs – 3-4_minCMV-4xLexA:eYFP, 1-2_Hef1a:rttA3, 1-2_Hef1a:eBFP2
~10AM: Minipreps done on the 6 LRs that succeeded from yesterday by Grant
Nanodrops
Date
|
Assignee
|
Vector
|
Concentrations (ng/uL) *denotes 260/280 below 1.8 |
6.14 11AM
|
Jon/Michelle/Clara
|
2-3_Tre:C1434-VP16
|
A: 284.0* (1.78), B: 391.3, C: 380.9, D: 205.5 |
6.14 11AM
|
Jon/Michelle/Clara
|
2-3_Tre:LexA-VP16
|
A: 361.4, B: 193.9* (1.64), C: 212.9* (1.58), D: 390.6 |
6.14 11AM
|
Jon/Michelle/Clara
|
4-5_Tre:mKate
|
A: 176.9, B: 180.3, C: 188.7, D: 208.8 |
6.14 11AM
|
Jon/Michelle/Clara
|
3-4_minCMV-4xC1434:eYFP
|
A: 173.8, B: 152.5* (1.60), C: 319.5, D: 192.5 |
6.14 11AM
|
Jon/Michelle/Clara
|
3-4_minCMV-7xMnt1:eYFP
|
A: 147.7, B: 251.5, C: 147.6, D: 178.2 |
6.14 11AM
|
Jon/Michelle/Clara
|
3-4_minCMV-4xMnt1:eYFP
|
A: 195.1, B: 241.2* (1.75), C: 193.7, D: 157.0 |
Restriction Maps, 6.14 5PM
Assignee
|
DNA
|
Enzyme
|
Expected Results
|
Comments
|
Jon
|
3-4_minCMV-4xMnt1:eYFP
|
SalI-HF
|
5288bp, 2044bp if works; 5288bp, 1600bp, 977bp if doesn't |
All: 1uL SalI, 2uL Buf 4, 2uL BSA; A,B,C: 3uL DNA, D: 4uL DNA; DI up to 20 uL
|
Jon
|
3-4_minCMV-7xMnt1:eYFP
|
SalI-HF
|
5288bp, 2257bp if works; 5288bp, 1600bp, 977bp if doesn't |
All: 1uL SalI, 2uL Buf 4, 2uL BSA; A,B,C: 3uL DNA, D: 4uL DNA; DI up to 20 uL |
Jon
|
4-5_Tre:mKate
|
SalI-HF
|
5288bp, 1995bp if works; 5288bp, 1600bp, 977bp if doesn't |
All: 1uL SalI, 2uL Buf 4, 2uL BSA; A,B,C: 3uL DNA, D: 4uL DNA; DI up to 20 uL |
Mariola
|
2-3_Tre:C1434-VP16
|
SalI-HF
|
5288, 1862, 412 bp if works; 5288bp, 1600bp, 977bp if doesn't |
All: 1uL SalI, 2uL Buf 4, 2uL BSA; A: 1.8uL DNA, B,C: 1.3uL DNA, D: 2.4uL DNA; DI up to 20 uL |
Mariola
|
3-4_minCMV-4xC1434:eYFP
|
BamHI
|
3757, 1855, 1166, 478 bp if works; no cut if doesn't |
All: 1uL BamHI, 2uL Buf 4, 2uL BSA; A 2.9uL DNA, B: 3.2uL DNA, C: 1.6uL DNA, D: 2.6uL DNA; DI up to 20 uL |
Tyler
|
2-3_Tre:LexA-VP16
|
ScaI
|
2 cuts: 4464 and 2729 bp if works; 3 cuts: 3535, 2729, 1589 if doesn't |
All: 1uL SalI, 2uL Buf 4, 2uL BSA; A: 3uL DNA, B,D: 1.5uL DNA, C: 2.5uL DNA; DI up to 20 uL
From Gel Below:
A and C show the entry vector, B failed as well, D seems to have worked. There is uncut DNA around 7000 bp, but the other bands seem correct. D will be sent for sequencing. |

Midipreps were done by Kenneth and Tyler for sequenced verified constructs, 2-3_Tre:LacI-Krab, 3-4_minCMV 1xCI434:eYFP, and 3-4_Hef1ALac0:eYFP. Unfortunately DNA yields were low, as shown in the table below, so minipreps will be performed tomorrow, June 16, 2011.
| Date |
Assignee |
Vector |
DNA Concentration (ng/uL) |
| 6.15.2011 |
Tyler |
2-3_Tre:LacI-Krab |
54 |
| 6.15.2011 |
Kenneth |
3-4_minCMV 1xCI434:eYFP |
216 |
| 6.15.2011 |
Kenneth/Tyler |
3-4_Hef1ALac0:eYFP |
failed |
June 16, 2011
~10am Minipreps
| Date |
Assignee |
Vector |
DNA Concentration (ng/uL) |
| 6.16.2011 |
Tyler |
2-3_Tre:LacI-Krab |
509.1 |
| 6.16.2011 |
Kenneth |
3-4_minCMV 1xCI434:eYFP |
89.1 |
| 6.16.2011 |
Kenneth/Tyler |
3-4_Hef1ALac0:eYFP |
385 |
June 17-18, 2011 Construction
Protocol for all Cells: Added DNA 5 minutes after removing cells from freezer. Incubated for 30 minutes. Heat shocked for 30 seconds at 42 Celsius. Incubated on ice for two minutes. Added 1 mL of SOC. Grew at 30 C for 2 hours. Plated 100 uL from total reaction on one half of plate & the rest of the reaction on the other half of plate.
Date
|
Assignee
|
DEST_R4R2
|
Colonies (low/high conc.) |
6.18.2011
|
Grant |
pEXPR12-Hef1A-eBFP2 |
Order of <10 |
6.18.2011
|
Grant |
pEXPR12-Hef1A-rtTA3 |
Order of <10 |
6.18.2011
|
Grant |
pEXPR23-TRE-CI434.VP16 |
Order of 100-1000 |
6.18.2011
|
Grant |
pEXPR23-TRE-LexA.VP16 |
Order of 100-1000 |
6.18.2011
|
Grant |
pEXPR23-TRE-Mnt.VP16 |
Order of 100-1000 |
| 6.18.2011 |
Grant
|
pEXPR34-minCMV.4xCI434-EYFP |
Order of 1000-10000 |
| 6.18.2011 |
Grant |
pEXPR34-minCMV.4xLexA-EYFP |
Order of 1000-10000 |
| 6.18.2011 |
Grant |
pEXPR34-minCMV.4xMnt-EYFP |
Order of 1000-10000 |
| 6.18.2011 |
Grant |
pEXPR34-minCMV.7xMnt-EYFP |
Order of 1000-10000 |
| 6.18.2011 |
Grant |
pEXPR45-TRE-mKate |
Order of <10 |
Conclusion: pDEST12 appears to be growing slowly in all cases, as does pDEST45. pDEST23 grows at the rate typically expected by Gateway reaction standards while pDEST34 grows at a rate much faster than these standards. Corroborated in Weiss lab?
Week 3: June 20 - June 24
In the third week we continued expanding our library of usable DNA parts by creating more combinations of genes and promoters using the LR reaction.
June 20, 2011
Minipreps and nanodrops done on the plasmids from yesterday by Clara and Grant.
Restriction digesting done on the 10 transformations.
Assignee
|
DNA
|
Enzyme
|
Expected Results
|
Picture of Gel
|
Time Incubated
|
Comments
|
Jon
|
1-2_Hef1a-rtTA3 |
SalI-HF
|
5288, 2877bp if works; 5288, 1600, 977bp if it doesn't
|
Gel 1 Lanes 2-4
|
2 hrs
|
Being redone
|
Jon
|
1-2_Hef1a-eBFP2 |
SalI-HF
|
5288, 2889bp if works; 5288, 1600, 977bp if it doesn't |
Gel 1 Lanes 5-7
|
2 hrs |
Being redone |
Jon
|
3-4_minCMV-7xMnt1:eYFP |
SalI-HF
|
5288bp, 2257bp if works; 5288bp, 1600bp, 977bp if doesn't |
Gel 1 Lanes 8-10
|
2 hrs |
A and C sent off for sequencing
|
Jon
|
2-3_Tre_Mnt1-VP16
|
ScaI
|
4440, 2729bp if works; 3535, 2729, 1589bp if doesn't
|
Gel 1 Lane 11, Gel 2 Lanes 2-3
|
2 hrs |
A sent for sequencing; also being redone
|
Michelle
|
4-5_Tre_mKate
|
SalI-HF |
5288bp, 1995bp if works; 5288bp, 1600bp, 977bp if doesn't |
Gel 2 Lanes 10-11, Gel 3 Lane 2
|
2 hrs |
B sent off for sequencing; also being redone
|
| Michelle |
2-3_Tre:CI434-VP16
|
SalI-HF |
5288, 2270bp if works; 5288, 1600, 977bp if doesn't
|
Gel 2 Lanes 7-9
|
2 hrs |
Being redone |
| Michelle |
2-3_Tre:LexA-VP16 |
SalI-HF |
5288, 1913bp if works; 5288, 1600, 977bp if doesn't |
Gel 2 Lanes 4-6
|
2 hrs |
B sent off for sequencing
|
Charles
|
3-4_minCMV 4xLexA:eYFP |
SalI-HF |
5288, 1849bp if works; 5288bp, 1600bp, 977bp if doesn't |
Gel 3 Lanes 6-8
|
2 hrs |
B sent off for sequencing; also being redone
|
Charles
|
3-4_minCMV 4xMntI:eYFP |
SalI-HF |
5288bp, 2044bp if works; 5288bp, 1600bp, 977bp if doesn't |
Gel 3 Lanes 3-5
|
2 hrs |
Being redone |
Charles
|
3-4_minCMV 4xCI434:eYFP |
SalI-HF |
5288, 1960bp if works; 5288bp, 1600bp, 977bp if doesn't |
Gel 3 Lanes 9-11
|
2 hrs |
B sent off for sequencing
|

Key: Ladder, 3x Hef1a rtTa3, 3x Hef1a eBFP2, 3x minCMV 7xMnt1:eYFP, Tre Mnt1 VP16 A, Ladder; Tre Mnt1 VP16 B+C, 3x Tre LexA VP16, 3x Tre CI434 VP16, Tre mKate A+B, Ladder

Key: Ladder, Tre mKate C, 3x minCMV 4xMnt1, 3x minCMV 4xLexA, 3x minCMV 4xCI434, Ladder
June 23, 2011
Colony Counts
These results are based on plating 100 uL of the outgrowth culture.
Part Shorthand
|
Colonies this Time
|
Average Colonies Before
|
Notes
|
TRE:mKate
|
~10
|
~3
|
Using new TRE, Dest 23.
|
TRE:MntVP16
|
~50
|
~10
|
Using new TRE, Dest 23.
|
TRE:cI434VP16
|
~30
|
~1
|
Using new TRE, Dest 23.
|
Hef1A:rtTA3
|
~50
|
~1
|
Using new Hef1A, Dest 23.
|
Hef1A: eBFP2
|
~30
|
~3
|
Using new Hef1A, Dest 23.
|
4xLexA:eYFP
|
~1000s
|
~1000s
|
Using Dest 23.
|
4xMnt:eYFP
|
~1000s
|
~1000s
|
Using Dest 23.
|
Conclusion: pENTR vectors probably weren't properly aliquoted the first time around, strongly reducing recombination efficiency.
Week 4: June 27 - July 1
In the fourth week we implemented a color-coded box system in our -20 freezer to accommodate for the growing need of organization of the growing number of DNA parts. We began learning and using the Gibson reaction to create the gene part AVPR2-TEVs-GV16, which is expressed to produce a vasopressin receptor bound to Gal4-VP16 by a TEV sequence that can be recognized by the TEV Protease. Gibson reaction results were not great, and it appeared that our AVPR2 DNA was not of sufficient quality, so we re-PCRed the AVPR2 DNA segment.
June 28, 2011



June 29, 2011
~ Miniprepped at 10:30 AM
Date
|
Assignee |
Vector
|
DNA Concentration
|
6.29.2011
|
Mariola
|
pENTR_UAS
|
A: 98.7 ng/ul
B: 88.1 ng/ul
|
6.29.2011
|
Mariola
|
pENTR_Tet-LacO
|
A: 84.7 ng/ul
B: 83.2 ng/ul
|
6.29.2011
|
Jenny
|
pENTR_TEV-Protease
|
A: 97.5 ng/ul
B: 50.4 ng/ul
|
6.29.2011
|
Jenny
|
pENTR_LacI-miRRF4
|
A: 58.2 ng/ul
B: 161.4 ng/ul
|
6.29.2011
|
Michelle
|
pENTR_GV16
|
A: 49.9 ng/ul
B: 52.9 ng/ul
|
June 30, 2011
11:45AM ~ 12:45PM (Charles, Clara)
Miniprep AVPR2 for TANGO: 270.9 ng/ul (successful)
3:00PM ~ 5:00 PM (Charles, Clara)
PCR primer # 13, 14, 17, 18, 19, 20
- AVPR2 - TEVs
Annealing temperature: 58.9 C (13 - 58.6 C; 14 - 55.9 C)
Extension time: 18 seconds (~1200 bp)
- Gal4 - VP16
Ta: 62.4 C (17 - 62.4 C; 18 - 59.4 C)
Ext: 13 seconds (~740 bp)
- L1L2 Backbone
Ta: 65.4 C (19 - 62.4 C; 62.4 C)
Ext: 30 seconds (~2500 bp)
5:00PM ~ 5:30PM (Charles, Clara)
Gibson assembly cont'd: inoculated and incubating samples overnight
July 1, 2011
| Time |
Notes |
| 8:00 AM |
Color-Coded inventory implemented in the -20 freezer.
To be implemented in the -80 freezer. See below for details. |
| 9:00 AM |
Morning meeting. |
| 11:00 AM |
Design meeting. Significant amount of information to be
added to wiki in terms of construction plans and proposed
circuits. Do ASAP. |
| 1:30 PM |
LR reactions of pDEST_45, pENTR EYFP-FF6, and the five
inducible minCMV promoters complete. |
| 2:00 PM |
Kenneth's next PCR of Delta started. |
| 2:00 PM |
Cell stocks of the working pEXPR transactivators, TRE:mKate,
and Hef1a:EBFP2 made. |
| 2:00 PM |
Inoculation of the aforementioned pEXPR vectors for later
transfections completed. |
1:00 PM ~ AVPR2 - TEVs - Gal4 VP16 cont'd:
Clara did PCR purification and nanodrop to check the concentrations.
The concentrations were relatively low:
AVPR2: 20.8 ng/uL
Gal4-VP16: 30ng/uL
L1L2 Gibson: 16 ng/uL
Used 10uL of each sample to run the gel.
The following are the pictures of the gel (Same picture but different exposure levels), in order of AVPR2; Gal4-VP16; and L1L2 Gibson:
Expected Length - AVPR2: ~1200 bp; Gal4-VP16: ~740 bp; L1L2 Gibson: ~2500 bp


Gal4-VP16 and L1L2 Gibson seem to be successful. Should PCR AVPR2 again.
As for the Gibson assembly practice, the practice samples were over-incubated so I had to throw them away. (Sorry, Charles!)
Color-Coded Inventory implemented in the -20 freezer.
Purple: Destination Vectors
Red: Gene Vectors
Green: Promoter Vectors
Yellow: Source/Ordered Vectors
Orange: Expression Vectors
Blue: Primers (5 uM)
Pink: Primer Stocks (100 uM)
Not yet ported to the -80 freezer cell stocks.
Week 5: July 4 - July 10
We began looking into using the Goldengate assembly method, but two gene elements contained a cut site that needed to be mutated out. We performed Site-Directed Mutagenesis using the Lightning Kit in the hopes of mutating out the cut site, but results were not successful due to mishandling during the protocol, so the procedure was set to be repeated. Gibson assembly of AVPR2-TEVs-GV16 continues as we re-PCRed the AVPR2 DNA segment and re-run the entire Gibson protocol, picking 20 colonies in a determined attempt to obtain a successful result.
July 10
Jenny and Jon - SDM Colony counts were approximately 50 for the control, 20 for the TEV, and 0 for the Gal4VP16. There is a chance the TEV and Gal4VP16 labels were switched, which we'll find out after sequencing. Six colonies were picked from the TEV plate and inoculations were accidentally run for 22 hours at 37C.
July 9
Jenny and Jon redid the SDM for Control, Gal4VP16, and TEV using the Lightning Kit, following pages 7 and 8 of this manual: www.qcbio.com/stratagene/210518.pdf
We used 1.5uL 100mM stock of the previously designed primers. The final reaction volume was only approximately 45uL, as we only had about 5uL of 5ng/uL dsDNA for both the Gal4VP16 and TEV reactions. We got the DNA from the dilutions that Divya and Tyler previously used.
For transformation, we followed the standard protocol instead of the one outlined in the manual and outgrew at 37C and incubated at 30C for 16 hrs.
July 8, 2011
| Sample |
Assignees
|
Procedures
|
Enzyme(s) Used
|
Expected Bands
|
Results
|
EXPR 1xCI434 (A-C)
|
Grant/Michelle/Charles
|
Miniprepped
Nanodropped
Restriction Digest
|
ScaI
|
5000
2000
|
|
EXPR 4xCI434 (A-C)
|
Grant/Michelle/Charles
|
Miniprepped
Nanodropped
Restriction Digest
|
ScaI
|
5000
2000
|
|
EXPR 4xLexA (A-C)
|
Grant/Michelle/Charles
|
Miniprepped
Nanodropped
Restriction Digest |
ScaI
|
5000
2000
|
|
EXPR 4xMnt1 (A-C)
|
Grant/Michelle/Charles
|
Miniprepped
Nanodropped
Restriction Digest |
ScaI
|
5000
2000
|
|
EXPR 7xMnt1 (A-C)
|
Grant/Michelle/Charles
|
Miniprepped
Nanodropped
Restriction Digest |
ScaI
|
5000
2000
|
|
EXPR UAS:EBFP2 (A-C)
|
Grant/Michelle/Charles
|
Miniprepped
Nanodropped
Restriction Digest |
ScaI
|
5000
2000
|
|
EXPR AVPR2 A (1-3)
|
Grant/Michelle/Charles
|
Miniprepped
Nanodropped
Restriction Digest |
NcoI
|
1356
3000
|
|
EXPR AVPR2 B (1-3)
|
Grant/Michelle/Charles
|
Miniprepped
Nanodropped
Restriction Digest |
NcoI
|
1356
3000
|
|
EXPR AVPR2 C (1-3)
|
Grant/Michelle/Charles
|
Miniprepped
Nanodropped
Restriction Digest |
NcoI
|
1356
3000
|
|
EXPR AVPR2 D (1-3)
|
Grant/Michelle/Charles
|
Miniprepped
Nanodropped
Restriction Digest |
NcoI
|
1356
3000
|
|
July 7, 2011
Charles and Clara @ Knight lab: All four Kan plates had colonies, 40-50 colonies except for one of Deepak's gibson mix reactions, which had ~400 colonies. Inoculated 5 colonies from each reaction into LB-KAN. 20 Falcon tubes in 37C spinning. To be taken out at 2 AM by Grant.
July 6, 2011
Charles and Clara @ Weiss lab: Ran Gibson reaction with both DM and CH Gibson mixes each, total of 4 reactions run. 0.21 uL of 25.6 ng/uL AVPR2, 0.11 uL of 30 ng/uL GV16, 0.73 uL of 16 ng/uL L1L2 backbone added to each 15 uL Gibson mix. Incubated in thermocycler at 50 C for 1 hour. (Used formula to obtain required volume for 7 fmol: 7 x 0.65/(conc ng/uL) x length / 1000.
Charles and Clara @ Knight lab: Gibsons transformed into 10G cells (0.9mL SOC), incubated spinning for 1 hour at 37C. Centrifuged for 3 min max speed (14000), removed 500uL of supernatant, resuspended the remaining & Plated 100 uL of each tube on KAN. Incubating plates 37C overnight (6:30pm ~ right after morning meeting tmr). The leftover tubes (Gibson + transformation) are in the iGEM fridge.
Divya and Tyler's SDM:
We used the Agilent QuikChange II SDM Kit and followed the protocol in its manual (http://www.genomics.agilent.com/files/Manual/200523.pdf).  ;
;
GV16 Mutagenesis (to remove BsaI site)
| Amount of dsDNA |
10 ng [A] |
20ng [B] |
50ng [C] |
| dsDNA template |
1.89 uL |
3.78 uL |
9.45 uL |
10x Reaction Buffer
|
5 uL |
5 uL |
5 uL |
| GV16 Mutagenesis Primer (Forward) |
1.25 uL |
1.25 uL
|
1.25 uL
|
| GV16 Mutagenesis Primer (Reverse) |
1.25 uL
|
1.25 uL
|
1.25 uL
|
| dNTP mix |
1 uL |
1 uL
|
1 uL
|
| H2O (to get to 50 uL) |
39.61 uL |
37.72 uL |
32.05 uL |
Then added 1uL HF DNA Polymerase
TEV Protease Mutagenesis (to remove BsaI site)
| Amount of dsDNA |
10 ng [A] |
20ng [B] |
50ng [C] |
| dsDNA template |
1.98 uL |
3.96 uL |
9.92 uL |
| 10x Reaction Buffer |
5 uL |
5 uL |
5 uL |
| TEV Protease Mutagenesis Primer (Forward) |
1.25 uL |
1.25 uL |
1.25 uL |
| TEV Protease Mutagenesis Primer (Reverse) |
1.25 uL |
1.25 uL |
1.25 uL |
| dNTP mix |
1 uL |
1 uL |
1 uL |
| H2O (to get to 50 uL) |
39.52 uL |
37.54 uL |
31.58 uL
|
Then added 1uL HF DNA Polymerase
I accidentally added too much Primer to the 10ng tube, so it was scrapped.
After going through the Thermal Cycler, 1uL of DpnI was added to each remaining sample. The samples were then put in the incubator at 37 degrees C for 1 hour, then moved to the freezer.
July 5, 2011
AVPR2 re-PRC: Charles and Clara re-PCR-ed the AVPR2 using primers 13 and 14. This time, however, we used 20s extension time instead of 18s.
Tyler: Did LR at 4:30 pm for pEXPR 4-5 UAS:EBFP2. Will transform tomorrow morning. Plan on also getting LacI (without the Krab) from Weiss lab for use as a weak repressor.
<>
Week 6: July 11 - July 17
In the sixth week we continued re-attemping previous failures using modified protocols or higher quality DNA in hopes of obtaining successful reactions. We also began looking at Cadherins and the possibility of using them as a clumping mechanism for mammalian cells. In order to visualize Cadherins, we needed some sort of fluorescent color, so construction of NCadherin-EGFP (NCadherin is one of many types of cadherin) began. DNA for NCad-EGFP was ordered, but needed to be in a format such that we could LR react it with the different promoters we have. This is done by attaching attB sites to flank the NCad-EGFP gene and then performing the BP reaction, which generates LR reaction-compatible parts. PCR of the attB sites was successful. By this week we have also begun work with mammalian cell cultures. To explore the limitless possibilities of synthetic biology, a few of us took it upon themselves to look into other interesting gene components, such as the Caspase gene and the FF4 tag.
Week 7: July 18 - July 24
Part of the team worked on creating protocols for a robot liquid handler to run the usual lab reactions that we run. We are hoping that the robot can replace us and do our liquid-related lab work for us. Various dry test runs were done. We transfected various DNA parts that we have into Hek293 cells, and results show that most of our DNA works. We investigated the TRE-rtTA3 system as well as the UAS-Gal4 system. Both seemed to be functional.
Week 8: July 25 - July 31
This week we underwent a momentous drive to create more LRs. A large list of promoter-gene pairs was conceived of and we began to run through LR reactions in somewhat of a factory manner. This occupied much of our time. We also received and prepared DNA parts from Elowitz's group in Caltech. We also got trained on using the FACS machine and began to get quantitative data on our transfections.
Week 8
h3. July 31
Kenneth: passed cells for transfections tomorrow. Planned out a series of experiments. Imaged cells from 7/29 transfection with free delta. also did FACS stuff.
Mariola: FACS of Testing UAS:LacIKRAB (day 2), and of Knockdown Pulse (day 1). Also imaged Pulse. #tired
Grant: Did 14 PCR reactions to generate parts for the CCR5-TEVs-GV16, HRH4-TEVs-GV16, and B-Arrestin-2-TEV-Protease ENTR vectors. Gel extracted all reactions, completed a Gibson reaction, and transformed the Gibson reactions. Miniprepped and restriction mapped several previous LRs, including four samples from Tyler's LRs (All of which were Notch-Gal4 under different promoters). Only the pEXPR_23_Hef1a-LacO_Notch-Gal4 construct had proper bands, and will be sent for sequencing. Inoculated quintuples of DRD2-TEVs-GV16 and pDisplay-CCL5-MYC. Was disappoint. [Ken and I noted a possible issue with the UAS promoter from Elowitz.|Sequence Alignments]
h3. July 30
Kenneth: Prepped samples for FACS. added free delta to cells. Passed more cells.
Grant: Did something. Forgot what. Probably inoculations, miniprepping, and restriction mapping, since I discovered that pDisplay-IL8-MYC looks good.
Mariola: FACS of Mnt and CI434 and Testing UAS: LacIKRAB (day 1) experiments. (6pm) Added Shield, Dox and Free Delta in various combinations to 3 INPUT AND Gate (8pm), Cocultured cells for Knockdown Pulse. #funfunfun
h3. July 29
DNA Team tasks (put comments below each task):
\- Divya 7/26 LRs (6 w/colonies): Nanodropped and restriction digested by \____. Good samples sent for sequencing by \_____.
\- Divya 7/26 LRs (7 re-trans): Miniprepped in afternoon by \____. Nanodropped and restriction digested by \_____.
\- Tyler 7/28 LRs (4): Transformed in morning by 12. Imaged cells and updated wiki (See [Monoculture with Cis-inhibition|iGEM2011:Monoculture with Cis-inhibition] and [iGEM2011:TRE.GAL4 UAS Activation]). Planned transfection for tomorrow (needed to wait for hef1A:rtTA)
\- Promoter propagations + NCad-EGFP BPs: Good restdig BPs sent for sequencing by \_____. Cell stocks of good restdig promoters made by \_____.
\- Re-acquired 4 Weiss Plasmids by \_____.
Grant: Did extensive lab cleanup (note: should look the same by the end of the day. If it doesn't, consequences will ensue\!). Inoculated four new parts (one LR and three Gibson reactions\- pEXPR_23_Hef1a-LacO_TEV-Protease, pDisplay-CCL5, pDisplay-IL-8, and DRD2-TEVs-GV16, respectively). Made six gels. Sent nine samples off for sequencing after finishing analysis of the restriction map I ran in the early morning. Assisted with the deluge of lab questions, as always.
_Note: Colony counts were roughly 10, 50, 50, and 50, respectively. Gibson reaction was conducted at 52 C rather than 50 C due to previously low or zero colony counts; this may be worth generalizing to future Gibson reactions, assuming 40 bp of overlap. _
Clara: restriction digested 11 samples (x 2 each). Incubating in 37C 1:10 ~ 4:10. See [iGEM2011:Clara's Notebook] for more details.
Michelle: Miniprepped 19 samples that Divya had LRed or that were received from the Weiss Lab.
Kenneth: Sent off Hef1a-lacO:pdisplay-vaso-myc for sequencing. Also did second set of transfections to test free delta activation of Notch.
Mariola: Aliquoted DNA. Nanodropped. Helped miniprep? I don't remember anymore. Transfected miRNA Knockdown Pulse, 3 input AND gate. Imaged Mnt and CI434. Got SHIELD from Noah. 1ug/mL to fully stabilize.
h3. July 28
DNA Team Sub-tasks:
\- Divya 7/26 LRs (13): 6 plates w/ colonies, GR inoculated, CH miniprepped. 7 plates w/ colonies, CH re-transformed, GR to inoculate midnight.
\- Divya 7/27 LRs (4): CH transformed morning, GR to inoculate midnight.
\- Promoter propagations + NCad-EGFP BPs: Miniprep MD, CP. Restriction digested night GR.
\- Make plates + acquire ethanol: DA, CP.
\- Tyler 7/28 LRs (4): LR-ed TW afternoon. Tyler also added Dox to transfections from yesterday and imaged cells.
\- EXPR-Tyler propagations: completed miniprep CH, high concentration stock, will last on average \~5 experiments.
Charles: Transformed Divya's 4 LRs started on 7/27. Used 1.5 uL of each LR reaction to transform. Re-transformed the 7 of Divya's 7/26 LRs that did not have any colonies. Started miniprepping propagations EXPR-Tyler plasmids.
Grant: Inoculated Divya's LRs started on 7/26. 7 out of 13 plates had no colonies. The 6 that had colonies had fewer than 10 colonies, may possibly be background.
Jon: pEXPR_2-3_UAS_amCyan-miRFF4 looks good based on sequencing results. Made a cell stock and put it into the \-80C. Also made cell stock of the pEXPR_2-3_TRE_Caspase3 despite sequencing issues, as the sequence came back consistent. Appears to be two forward TEV protease primers in new SDM, so it's being put on hold temporarily.
Kenneth: Mainly tissue culture work. Imaged cells and prepped for FACS. Added free delta to wells. Working on planning future experiments. Will pass cells and try experiments again with improved protocols.
Mariola: Passaged cells. Cocultured UAS: LacIKRAB experiments. Induced Mnt and CI434 with Dox. #lotsofwork
Michelle: Miniprepped promoter propagations and BP reactions with Clara, worked on other minipreps with Jenny, and helped Charles transform LRs.
h3. July 27
Kenneth: Sent off sequencing samples for last of LR's in personal pipeline. Miniprepped hef1a-lacO:pDisplay-vaso-myc LR. Restriction digested samples with EcoRI. Gel looks good, all three samples worked. Aliquotted DNA for large numbers of transfections today. Number of experiments lined up for imaging and stimulation tomorrow.
Mariola: Transfected Testing:UAS LacIKRAB, Mnt (Attempt #2) and CI434 Parts Characterization Experiments.
Grant: Aligned sequencing results and assisted sequencing. Inoculated additional pDisplay-Myc-Vasopressin and two AVPR2-TEVs-GV16 attempts via Golden Gate. Did an LR of pEXPR_23_Hef1a-LacO_TEV-Protease for a future Tango test. Spun down samples from the day at 5 AM and inoculated colonies from six of the 13 LR reactions (all plates that had colonies had very low colony counts between 1 and 7 colonies per plate). Finalized entries for the [Gene Synthesis Challenge].
Clara: Transformation for Divya's LRs (numbered 1-19) from yesterday, except for the ones that were incomplete due to lack of promoters (# 1, 2, 5, 10, 14, 15). Total of 13 plates are being incubated at 37 C, 2:30 pm ~
DNA Team: Inoculated transformations from 7/26 of BP reactions, minCMV-1xCI434, minCMV-4xCI434, Hef1a, and minCMV-7xMnt 12 PM (Jon). Transformed LRs from 7/26, plated 37C at 2:30 PM (Clara). Miniprepped propagations, low yield (Michelle, Mariola). Cell stocks of 7 samples including UAS citrine, CMV-Notch-Gal4 1 and 2, CMV-TO Delta-mCherry 1 and 2, and UAS mKate to be made by Divya. MIA Weiss plasmids also to be tracked down by Divya.
Charles: Worked with members on above tasks. Inoculated Tyler's requested EXPRs around 12 PM.
Divya/Michelle: Made cell stocks of samples provided by Deepak and Charles. Prepped samples for sequencing. Did LRs which Tyler had requested. Searched for missing plasmids which had been provided earlier by Deepak. It turns out Grant/Ken had disposed of them during an earlier cleaning session. I haven't been able to find any duplicates, so all 4 will have to be re-made. (See Divya's Personal Notebook for details). LRs will be transformed tomorrow morning. Still need to make more promoter entry vectors for those that were unavailable for yesterday's LRs.
Jon: Inoculated 22 colonies from yesterday's transformations. Michelle miniprepped two new cell growths from yesterday, so I prepped those and sent them off for sequencing. Also helped Divya prep for sequencing. Defrosted gel room fridge and set up new burn box/arranged for pickup of old one. Will update colony counts tomorrow (sheet in lab)
Tyler - aliquotted DNA for transfections. Did transfections.
Louis - Pyevo stuff. Downloaded new version.
Jenny - [FACS images - 3 Color Experiment|FACS Images - 3 Color Experiment 7-26]
Sam - Started systematically recording videos of simulations of various circuits. Cleaned up CC3D code so others can understand it.
h3. July 26
Kenneth: inoculated 3 colonies from 1-2 Hef1a:display-vasopressin LR. sent out sequencing samples for previous LR's. Transfected CHO cells. Thawed out new Hek293 cells free of contamination. prepped cells for a bunch of notch delta cis inhibition characterization experiments tomorrow.
DNA Team accomplishments: Miniprepped a large number of DNA propagations, restriction digested 4 re-transformed verified DNA that did not have cell stocks, completed Mariola's transfection DNA order, re-organized Expression vector box, combining aliquots to leave two final aliquots for each expression vector. Nanodrops done by Mariola, concentrations to be posted. Entry vector dilutions completed except for 4 samples \-not enough, and 4 samples low-concentration.
Charles - 6AM checked AVPR2 sequence. Genewiz read \~1.2 kb, GV16 perfect, and about 200 bp of solid AVPR2 read before sequencing died off. AVPR2 is successful, should sequence again with forward primer to verify complete sequence. 6 AM BP reaction on NCad-EGFP, duplicates done of each of three successfully gel extracted PCR reactions. Prepared massive miniprep of expression vector propagations. Introducing new workflow efficiencies. Completed Mariola's transfection DNA order.
Jon - pEXPR_2-3_TRE_rtTA-DD sequencing results look good\! Made a cell stock and put in the \-80C. pEXPR_3-4_TRE_Caspase3 sequencing results look very odd. Big region of partial match, but it looks like our Caspase3 sequence on Geneious is incorrect or our actual DNA is weird. Grew up C2 and E4 again (incubated at 4:20pm) to prep for sequencing as they have the potential to be good. Transformed 6 BPs, an expression vector, and 4 promoters for propagation.
{color:#000000}Grant - Random assistance throughout the day with questions. Reorganization of Geneious folders and freezer positions. Updating of wiki with information from sequencing results.{color}
Tyler - did minipreps of Elowitz Notch, Delta, Citrine, and also a UAS:mKate to be used for making cell stocks. Prepared cells for transfection. Would like to see FACS data for [iGEM2011:TRE.GAL4 UAS Activation] and discuss briefly tomorrow morning.
Louis - Got pyevo working. Read through pyevo several times. Attempted to implement a Transfer Labware command. Maybe it even worked.
Divya - Miniprepped 11 total samples, some were failed samples from last night plus a few more with Clara & Tyler. Set up new LRs. All are documented in {color:#003333}Divya's Personal Notebook{color}. (Thanks to Jenny for diluting the gene/promoter entry vectors\!) The FACS Team met with Deepak today (~5pm) to discuss data from yesterday.
Clara - Miniprepped 11 samples with Divya and Tyler. Restriction digest for UAS: mKate, CMV-Notch, CMV-TO: Delta-mCherry, UAS: citrine (X2 samples each). Restriction enzymes, expected lengths, buffer used are all logged in Clara's personal notebook. Restriction gel to be run by either Charles or Mariola later today.
Jenny - helped with DNA team. Updated [Computer Generated Circuits |iGEM2011:Computer Generated Circuits](fixed assumptions, etc.) Got FACs code from Deepak. Will generate graphs and Matlab functions in the morning.
Mariola - Passaged cells and planned out experiments for tomorrow's transfx. Ran restriction gel:
!mariola 7.26.JPG|border=1!
|| Lane || 1 \\ || 2 \\ || 3 \\ || 4 \\ || 5 \\ || 6 \\ || 7 \\ || 8 \\ || 9 \\ || 10 \\ || 11 \\ ||
| Part \\ | Ladder \\ | UAS:Citrine 1 \\ | UAS:mKate 1 \\ | CMV Notch 1 \\ | CMV-TO:Delta_mCherry 1 \\ | Ladder \\ | UAS: Citrine 2 \\ | UAS:mkate 2 \\ | CMV Notch 2 \\ | CMV TO dmC 2 \\ | Ladder \\ |
| Expected \\ | | 1 kb, \\
1.7 kb \\
3.8 kb \\ | 1.9kb \\
5.3 kb | 3.3 kb \\
8.5 kb | 400 bp \\
870 bp \\
2.2 kb \\
5 kb | | 1 kb, \\
1.7 kb \\
3.8 kb | 1.9kb \\
5.3 kb | 3.3 kb \\
8.5 kb | 400 bp \\
870 bp \\
2.2 kb \\
5 kb | |
h3. July 25
Kenneth - prepared the FACS samples. Also passed cells and dealt with possible contamination. Bleached all suspect containers. put all cells into quarantine incubator. Autoclaved all parts of contaminated incubator and thawed cells to grow in clean incubator. Proper separation of everyone's media and cells necessary to prevent spread of future contamination. Planned out proper separation of materials and protocol in for tissue culture to avoid widespread contamination. Also transformed LR of hef1alaco:display vasopressin. Updated wiki with LR pages, etc.updated wiki updated wiki updated wiki updated wiki
Tyler - prepared cells for FACS. Cells did not look healthy at all. Terrible transfection efficiency for tri-color experiment after transfection efficiency of almost 100% on previous Hef1A:rtTA3, TRE:Gal4-VP16, UAS:mKate experiment (see [iGEM2011:TRE.GAL4 UAS Activation]). Some interesting things happened with this experiment...Look at the increase in fluorescence (Note: same microscope settings were used). Also it looks like the UAS is leaky because even the control without rtTA had some background red. Helped Ken spray down the incubator with ethanol.
Grant - Did sequencing of eighteen samples and restriction mapped AVPR2 and other constructs. Transformed three AVPR2-TEVs-GV16 Golden Gate attempts, all of which yielded 50\+ colonies within 10 hours, and plated an additional LR attempt with pDisplay-Vasopressin. Made four additional cell stocks and continued reorganization of the 4C and \-20C freezers. Massive wiki updating (adding and fleshing out LR pages) with Ken.
Charles - Expression vector propagation. Michelle miniprepped yesterday's inoculations, then I refilled several tubes with LB-Amp to regrow again. Also inoculated 2 tubes each of CMV:Notch Gal4, CMV-TO: DmCh, UAS:citrine, UAS:mKate. Tons of colonies from each, should have plated a dilution. Some inoculations may actually have 2 colonies because they were hard to pick, and unintended colonies may have been accidentally scraped. Ran FACS on the tricolor experiment, Notch Delta experiment, and Gal4 experiments. Data looks somewhat consistent with expectations but nowhere near ideal. Further analysis to be done, but at a glance, partial success (ie not as much of some fluorescence as expected, but at least some was observed). NCad-EGFP gel extracted. Faint band at 5.5kb observed in all lanes corresponding to plasmid. Strong bands at 3.5 kb corresponding to expected PCR products. PCR was successful. Gel extraction
Mariola - Imaged Mnt Parts Characterization experiment. Created a page and posted the (frankly, mediocre) results. Tracked cell stocks of parts and updated Workflow accordingly. Ken and Tyler spoiled an important plot point of DEXTER for me.
Louis - Fought with pyevo and lost. Figured some things out but got hung up on an error I can't figure out. I'll be asking about that tomorrow. Also, some assorted lab cleanup.
Michelle/Divya: Miniprepped and nanodropped 25 samples for cell stock purposes. 16 LRs. Divya ran FACS on Tricolor, Gal4, and Notch-Delta experiments. Data will be analyzed with Deepak tomorrow.
Jenny/Jon - Nanodropped all set of 6 LR samples, restriction digested A-E with SalI; unfortunately, we did not have any enzyme that was good for F (out of ScaI). Almost all of the restriction maps looked good, so we picked a sample from each and sent it off for sequencing.
Restriction mapping: !July25RestrictionMap.JPG|border=1!
Lane contents are as follows:
Gel1: Ladder \| A3/A4 \| A5 \| B2 \| B3 \| B5 \| C2 \| C3 \| C5 \| D1 \| D2 \| Ladder
Gel2: Ladder \| D4 \| D5 \| E3 \| E2 \| E5 \| 3 \| 4 \| 5 \| 6 \| 7 \| Ladder
See 7/20 for the key. Sent off A5, B5, C3, D1, E3, and F2 off for sequencing.
Week 9: August 1 - August 7
In the ninth week we did a lot of internal re-organization to increase our overall efficiency in work. This involved re-organizing an internal wiki that we use for management of our available DNA as well as a log of transfections needed to be done. Lots of samples were FACS-ed, generating lots of results that we can make graphs out of. Many of our parts were successfully characterized.
Week 9
h3. August 7
Kenneth - A gazillion number of samples for FACS (actually only 21) for rtTA-DD experiment and TRE:mKate. Preliminary results: Hef1a:eBFP2 did not show up. Worked on MATLAB code for gating, finished prototype. Added dox to the TRE:EBFP experiment. Also "procured" formaldehyde and BSA for immunostaining. Fixed and blocked cells for staining tomorrow. Also passed 4 plates of cells using new technique which should fix the clumping issue that is leading to non-uniform transfection efficiency. The key is to dilute cells to 4x10^5 and then add 0.5 mL to well, as opposed to adding a small concentrated amount of cells into well, then diluting.
Grant - Finished the miniprep in the morning\- concentrations were typically around 150 ng/uL, good enough for transfection. Inoculations of the EPHB2, EFNB1, and CXCR1-TEVs-GV16 were done a little late in the day, but will still be miniprepped after the morning meeting on Monday. Restriction mapping not started\- once SalI-HF is in the lab, will commence. Designed new primers for Notch engineering.
Mariola: Prepped and FACSed.
Tyler: FACS
Divya: Ran FACS for Ken, Tyler, and Mariola.
h3. August 6
Kenneth - Prepared a ubergajillion number of samples for FACS. Actually just 8. Took 2 eternities. Actually just 30 minutes. Transfected TRE:EBFP2 experiment.
Charles - Almost forgot to write: miniprepped exactly one gajillion tubes. Actually just 38. Took exactly eternity. Actually just like 3 hours (running out of PE halfway sucks). FACS 4 PM to 5 PM. 39 samples done. Mariola had 40% transfection efficiency. Tyler's had around 10%. Ken's stuff had no fluorescence. Results not yet saved onto server, but saved on computer.
Grant - Completed gel extraction, Gibson ligation, and transformation of the EPHB2, EPNB1, and CXCR1 ENTR vectors; inoculated duplicates of the ten LR reactions and the pDisplay-CCL5-MYC Gibson redo. On schedule for miniprep of the 22 samples as well as late night restriction mapping of the same. Did major update of plasmids in Geneious\- we now have folders for easy LR construction and a list of pretty much every LR we've constructed (and then some).
Mariola: FACSed Delta characterization experiment. Induced TRE: EYFP experiment with dox ladder.
Tyler: FACS for some colors. DOX for some TRE:Laci-Krab. Transfections for other color combos we didn't have cells for yesterday.
h3. August 5
Kenneth - Transfected pDisplay-vaopressin expression vector; will do immuno-staining on Sunday. Added dox and shield to cells.
Grant - Completed alignments of all sequences sent yesterday; all Robot LR reactions failed while Divya had a 3/8 success rate and I had a 11/13 success rate. Based on alignment of the failed sequences, I suspect the minCMV promoters we are using may be compromised. Transformed eleven constructs (the ten LR reactions referenced yesterday and the pDisplay-CCL5-MYC Gibson redo), completed PCR reactions for EPHB2, EPNB1, and CXCR1 (twice....................), made five gels for mapping/gel extraction, and made cell stocks of all verified constructs from yesterday's sequencing.
Charles - Re-inoculated stuff from Aug 4. Wiki organization.
Clara - Made LB broth at Weiss lab, and 2 LB agar bottles are autoclaved and located at Deepak's place for later use when we need more plates.
Mariola: Transfected.
Michelle\- BBC application video and edits with Jon and Tiffany.
h3. August 4
Tyler - Imaged all of the tri-color cells before preparing them for FACS ([Tri-Color Experiment v2|iGEM2011:Tri-Color Experiment v2]). Note that Hef1A:mKate works. Used Matlab to generate histograms for 7.25.11 FACS data ([iGEM2011:TRE.GAL4 UAS Activation]). These experiments all failed because of bad TRE:GV16, but note a couple things: 1.) Even though it looks like we have amazing efficiency from the pictures, the best we got was about 60% 2.) UAS:mKate is relatively leaky (about 10% of cells). Edited wiki
Grant - Did 54 minipreps of 24 new LR constructs and eight propagations; sent one sample from each of the 24 LRs for sequencing. Cleaned up both lab rooms, consolidated propagation DNA, cleaned \-20 freezer/reorganized boxes. Redid the pDisplay-CCL5-MYC Gibson reaction at 55C based on previous success at this temperature. Started new LRs of multiple Tango and characterization parts, referenced [here|Printing Page]. Did significant in-silico cloning and database updating. Was yet again disappoint.
Charles - Major re-organization of the wiki. Did many inoculations. To be miniprepped today. (There's more)
Divya - Aliquotted DNA for transfection. Delivered presents from Weiss Lab. Helped Charles organize wiki.
Michelle\- Nanodropped ~ 25 samples for Grant, BBC application.
Mariola: Passaged Cells.
h3. August 3
Tyler - Did Tri-color transfections as well as TRE:LacI-Krab Repression of LacO experiments. Will need to FACS colors tomorrow with Charles and add Dox to LacI-Krab wells and FACS those on Friday. The TRE:GV16 was resequenced by Grant and failed, so all of my experiments from 8.2.11 found here ([iGEM2011:TRE.GAL4 UAS Activation]) failed as well, although we did prove that TRE:mKate works. Hopefully we can get a Hef1A:GV16 soon. I also passed cells and prepared cells for transfections tomorrow. I would like to find the FACS data that was piggybacked onto the first tri-color experiments. If you look at the link above, it seems like we had very good efficiency on 7.25.11, so I would like to see how that translated over to FACS. Can somebody get me those histograms?
Clara - Restriction digest & gel by robot attempt #1 with JBabb and Divya. 11 samples included variants of 3 DNAs--TRE:LacI, minCMV4xLexA:eBFP2, Hef1a:Notch. TRE and Hef1a looked good, but all 6 of minCMVs had consistent but different from expected band length. To see the details of restriction digest and gel picture, see [iGEM2011:Clara's Notebook].
Mariola - Transfected Hef1a-lacO:Delta-mCherry verification experiments. Fixed auto_facs.m code. Ran it and posted up FACS data for my experiments. Wiki yupdates. Took an awesome nap. Some more wiki updates.
Grant - Miniprepped eleven samples (eight propagations and three new constructs, the UAS-AVPR2-TEVs-GV16 plasmids) with Michelle. Inoculated 50 samples (duplicates of 25 different LR reactions) for miniprepping today. Sent CCR5-TEVs-GV16 and B-Arrestin-2-TEV-Protease for sequencing; both reads came back successful. Multiple LRs, including Tango system LRs, will commence today, along with restriction mappings of the 50 plasmids.
Michelle\- Helped Grant miniprep eleven samples, also miniprepped pDEST samples from Deepak. Restriction digested 8 samples (UAS AVPR2 A-C, Hef1a DeltamCherry, UAS Ncad, Tre Ncad). Charles ran gel. UAS Ncad/Tre Ncad were cut with old SalI that didn't work, will be redone today with the new SalI-HF. Worked on BBC application with Kenneth.
h3. August 2
Kenneth: planned out future experiments, did research on protocols. Went down to image cells and induce with delta and dox. It does seem that "our" UAS is not very leaky at all as seen with UAS:mKate. Of course, could be effect of citrine having high stability compare to mKate. Also, we have H2B citrine, thus explaining the nuclear signal we keep observing. I have the protocols from Adrian/Zhen for Ncad knockdown and subsequent western. Also we can test for pDisplay-vasopressin-myc on cell surface with my proposed Immunofluorescence protocol. Will image the delta-induced UAS:mKate cells tomorrow and FACS either tomorrow or day after. Also Captain America was mediocre. Really a formulaic superhero movie with uninteresting characters. Plot was lame, full of holes. Characters were all archetypes.
Tyler - Prepared DNA for transfection. Cells were not ready. Will transfect tomorrow for color experiments. Need FACS team to prepare for tri-color repeat Thursday. Added Dox to cells tranfected yesterday ([iGEM2011:TRE.GAL4 UAS Activation] \- see bottom). Did not see any UAS leakage with UAS:mKate. Ken suggested that our UAS is different for that Elowitz used with UAS:citrine. Need DNA team to make UAS:EYFP and UAS:EBFP2, as well as other color reporters. Also imaged monoculture cis-inhibition. Ali did a repeat. (see [Monoculture with Cis-inhibition|iGEM2011:Monoculture with Cis-inhibition]). It shows cis-inhibition.
Mariola - Lots of wiki updates. Outline protocol for Hef1a-lacO:Delta mCherry verification transfections. Aliquoted. However, cells were not confluent so transfections will occur tomorrow. Captain America was dope.
Clara - Transformed 4 LRs (by robot yesterday) and 8 of Divya's LRs. Plates are in 37 C from 3pm. One of Divya's LRs labeled DA 2 has to be re-LRed and transformed because I put too little DNA for transformation \:( Also made plates with Divya (3 bags of amp, 1 bag of kan).
DNA Team: Miniprepped propagations and Divya's inoculations, sent 7 LRs for sequencing, transformed Divya's new LRs, transformed Clara's robot LRs. Inoculated 9 EXPR propagations at 4:30 PM.
Charles: BP Reaction using 1.0 ul and 2.0 ul of BP clonase instead of 0.5 ul. Started at 5:30 PM.
Jon: Discovered that sequences sent off on Monday failed because the wrong primers were used, thereby saving Grant five to ten minutes of time aligning the sequences. <\- you're welcome? \:P
Prepped stuff for sequencing, walked it over to Genewiz. Did not see Captain America as was walking over to Genewiz :'(
Grant: Workflow was similar to yesterday. Miniprepped and restriction mapped fifteen samples (quintuples of B-Arrestin-2-TEV-Protease, CCL5, and CCR5). Made TAE and additional gels for future restriction digestions. Transformed thirteen LR reactions and the redo of HRH4-TEVs-GV16. Saw Captain America.
Michelle: Miniprepped twenty-one of twenty-two samples (one failed to grow in liquid culture), most of which were propagations. Commanded Ali to aliquot LB. Saw Captain America.
h3. August 1
Kenneth: Rest Dig of Hef1a:eYFP-4xFF4. Also prepared samples for FACS for the free delta activation experiment part 2. Passed cells for transfection tomorrow. Also transfected set 3 of free delta experiment and co-culture, and TRE modulation of Delta
Grant: Miniprepped and restriction mapped quintuples of DRD2-TEVs-GV16 and pDisplay-CCL5-MYC. Sent off a lone sample of DRD2-TEVs-GV16 that appeared to map correctly for sequencing, along with pDisplay-IL8-MYC. Inoculated quintuples of CCR5-TEVs-GV16 and B-Arrestin-2-TEV-Protease Gibsons; HRH4-TEVs-GV16 did not form colonies and a Gibson was redone at a different temperature. Aliquoted or diluted various entry vectors and completed thirteen of twenty-one planned LRs before running out of LR Clonase.
Mariola: @ Woods Hole Oceanographic Institute with EBICS REU touring microtubule and cytoskeletal filament research laboratories. Delegated FACS of 3 Input AND Gate and Pulse (day 2) to Kenneth (thank you\!). Will play around with FACS data processing Matlab code and post images of experiments (~40 pictures) and update wiki tomorrow. Call me if questions arise. #shenanigans
Tyler: Transfected cells for Gal4 experiments, also repeated cis-inhibition experiment with just Delta and Notch. Added Dox to monoculture cis-inhibition experiment. Prepared cells for transfection tomorrow. Hopefully we have Hef1a:GV16.
Jenny - DNA Team stuff. [FACS images|iGEM2011:FACS - 7-31-2011].
Michelle/Divya: Nanodropped, restriction mapped and digested samples that were miniprepped on Friday (LRs and TEVs stuff). Charles ran the gel for these digests.
Jon - DNA stuff, ran to Genewiz in 10 min\!
Clara - Ran 4 LR reactions by robot with JBabb and Loius at the Weiss lab. LR reactions are at room temperature since 6:30 pm and will be ready for transformation after 8 - 16 hours since then.
Charles - Ran gels of two sets of rest digs. Sent 1 set of good plasmids off for sequencing. Yet to send good plasmids from second set for seq, also need to send NCad-EGFP. Experiment table organization/planning. And other stuff all around the wiki. Updates everywhere
Tiffany - online interface for robot service almost up..
Week 10: August 8 - August 14
Computer simulations of the Notch-Delta interactions were presented in our group this week, and we became convinced of the possibility of creating self-patterning mammalian cells. On the DNA side of things, we are trying to create more and more DNA using miniprep, because midipreps have for some reason not been successful for us or the 2010 iGEM team. On the transfection side, a lot of new DNA parts were transfected, observed under the microscope, and FACS-ed to quantify their effect/behavior.
Week 10
h3. August 14
Charles - Miniprepped propagations of various things, good CP LRs, as well as re-inoculations of poor CP LRs. Nanodrops were all over hte place. Some of the re-inoculated CP LRs reached 500 ng/uL, which was weird.
Divya - Ran FACS on 50 samples for Tyler and Ken. Also, I now have Koch access\! Woohoo\!\!\!
Kenneth - Prepped FACS samples. Moved frozen cells to ln2
Grant - Today and yesterday did sequencing of Tango and other plasmids as well as propagations of a handful of failed plasmids / spinning down of cells for Charles' miniprep.
h3. August 13
Kenneth - Added delta to cells. Co-cultured AVPR and pDisplay Vasopressin cells. Froze down and passed the Elowitz CHO cell lines and put in \-80. Also passed wells and flasks.
h3. August 12
Kenneth - Transfected cells for AVPR2 experiment as well as another free delta induction experiment, this time with const. color gating.
Charles - Attended Ken's orientation session and did 1 transfection just to try things out. Also obtained my new culture of cells. Cell stocked CP LR 4,5,6,7,8,11,12, of which 4,5, and 7 are tentative (repeat submitted). Re-inoculated failed CP LR's 1,2,3,9,10 with a different colony. Also propagated a whole bunch of plasmids, including the successful and tentatively successful CP LR's. Hopefully the color palette will be complete by Tuesday.
Jon - Tissue culture orientation & inoculated SDM transformations from yesterday. Colony counts around 20 for TEV and 3 for GV16.
h3. August 11
Jon - Redid GV16 and TEV SDM with new primers; transformed, hopefully they work this time.
Clara: Miniprepped and nanodropped 12 LRs from Aug 9. Hef1a: Delta-mCherry has esp. low concentration. To see conc. of other samples, refer to 8/11 [iGEM2011:Clara's Notebook]
Tyler: Prepared cells for transfection tomorrow. Plan on doing the following experiments: NCAD phenotype, TRE:LacI and TRE:LacI-Krab with proper gating, Hef1A:GV16 with proper gating, DRD2 repeat, and possible CCL5 experiment. Did FACS on [iGEM2011:Confluency Experiment] which shows best starting value around 1.5*10^5 cells/mL starting concentration. FACS machine showed around 40% efficiency. Matlab shows around 30%. In either case, we should start transfections with cells less confluent than we have been. TRE:LacI-Krab experiment seems to have worked, but there is only about 2x repression (see bottom of [Tri-Color Experiment v2|iGEM2011:Tri-Color Experiment v2]). Also put up FACS from AmCyan which is more green than blue.
Michelle: Prepped Clara's 12 minipreps for sequencing. Worked on Circuit Diagrams and write ups for the public wiki.
Charles: 42 FACS in less than 1 hour. Very fast because Ken's samples were concentrated.
h3. August 10
Tyler: I updated the wiki (first (per usual(winning))). Prepared for FACS. Transfected Confluency Experiment ([iGEM2011:Confluency Experiment]). Will FACS tomorrow. Still need FACS data from Divya for Tre:LacI-Krab experiment and some AmCyan colors. Today Jenny FACSed some DRD2 (apparently a failure from pictures) and some Delta-Notch stuff (also a failure). Need to look at the FACS, which apparently disappeared today? so hopefully we can find that tomorrow as well. Will try DRD2 (+100 uM dopamine) again later in the week with possibly more dopamine.
Charles: Completed Tyler's Hef1a_GV16 page with pretty FACS images. Watched Jenny do FACS at Koch. Some problems with either no cells in sample or visibly clumped cells. Introducing a new page... [iGEM2011:Color Palette]
Michelle: Inoculated Clara's LRs. Ready to miniprep and send for sequencing tomorrow.
Grant: Miniprepped, restriction mapped, and sequenced the minCMV promoters and a few other genes/promoters. Started LR reactions for a few more Tango parts and the Ephrin parts. Shockingly did nothing else.
h3. August 9
Kenneth: Worked on MATLAB stuff more. Treated TRE:mKate and TRE:eBFP2 experiments with dox. Transfected for pDisplay immunofluoresence experiment again. Thawed out Elowitz cell lines. Froze down large numbers of HEK and CHO cells. Passed cells into 60 mm plates for N-cad knockdown experiment. FACS data analysis. Prepared dopamine aliquots and supplies for future experiments.
Grant: Removed propagations of various gene/promoter entry vectors and inoculated a single colony from each. Did alignments of sequencing data for samples sent out yesterday. One LR reaction failed unexpectedly, but the other failures involving minCMV promoters appear to have occurred due to contamination from a Hef1a variant promoter based on a BLAST of the sequencing results. (READ: The "minCMV" promoter DNA is not actually minCMV promoter DNA\- at least this is the case for the tubes without stickers.) All parts currently being used for experiments are verified. On track for \~15-20 new LR reactions for Wednesday afternoon.
Clara: 12 LR reactions, left at room temperature @ 12:30pm~. Refer to [iGEM2011:Clara's Notebook] for details.
Charles: Transformed Clara's LR reactions. Had to leave during the middle of the day to go to the Student Services Office. Watched Mariola's presentation. Working on a new page.
Jenny: TriColor FACS are now up on their appropriate pages. Rest are temporarily on my notebook.
Tyler - Updated the wiki. Need to redo all of the FACS data tomorrow that Jenny did today because the red and blue colors are switched. Mariola's program works so I will use that. Tomorrow I will do transfections of a consistuitive color into wells with different confluencies that I set up today. I added Dox and Dopamine to wells today that we will FACS tomorrow.
Michelle/Divya: Miniprepped and nanodropped 12 samples. Prepped other samples for sequencing.
h3. August 8
Kenneth: planned out and did transfections for TRE eBFP2, TRE:mKate and AVPR2 validation experiments. Also threw in a co-culture of Notch/Delta cells, only with UAS:eYFP instead of UAS:citrine from Elowitz. Not expecting anything from that, but just covering all bases. Treated pDisplay-vasopressin-myc cells with anti-myc FITC Ab and prepared slide. Results suggestive. Cells were too confluent at time of fixation, many were washed off and the remaining look bad. Probably from my bootleg formaldehyde solution as well. However, remaining cells were green on the surface as well as red from delta-mcherry, while controls are not green. Seems to suggest it is being displayed...
Grant: Miniprepped the EPHB2, EFNB1, and CXCR1-TEVs-GV16 pENTR vectors assembled previously. Set up fourteen sequencing reactions of multiple Tango plasmids and a few reporter expression vectors designed to test the minCMV promoters, as well as the three ENTR vectors miniprepped. Pending affirmative sequencing results, LR reactions will begin. Because we need a number of promoters propagated and it would be nice to do LRs with the Rheoswitch system (and possibly the new Notch constructs) concurrently, I will probably wait to begin these. Cleaned the lab before the start of the work day, like a boss, or perhaps a janitor. Started propagations of a few promoters and genes that we had < 1 uL of DNA left of (and no cell stock), because if you want something done...
Tyler: Transfections
Charles: Propagated DNA we are low on. Looks like Grant also did this. So we should be stocked. And miscellanea.
Mariola: Aliquoted and Transfected UAS:EGFP and TRE:eyfp-4xFF4 experiments. Dox ladder of each. Writing 8pg REU paper and presentation.
DMC: Prepped and submitted several samples for sequencing. See Divya's Personal Notebook. Brought presents from Weiss Lab (SalI-HF, 10uL tips, 1000uL tips).
{cloak}
Week 11: August 15 - August 21
In our experimental attempt to characterize the Notch-Delta interaction, we used co-culture of CHO and Hek293 cells as well as stable cell lines of Notch-containing and Delta-containing cells from Elowitz to observe the trans-activation of Notch by Delta. Other experiments using CHO cells, which are more difficult to transfect than Hek293 using Lipofectamine, were carried out to observe the behavior of CHO transfected cells.
Week 11
h3. August 21
h4. Kenneth - Updates on Experiments:
Latest iteration of base FACS code: (incorporates Deepak's histogram update, removes the mean line on the histograms. Moves the quadrant lines to 200 for everything, since I've been seeing 200 as pretty much the max for the Blank samples. Can be easily changed.
[^auto_facsold.m] (the name is deceptive)
Results are in for dox ladder of Elowitz CMV-TO CHO cells [iGEM2011:Elowitz CHO Dox ladder], free delta induction of Elowitz receiver CHO cells. [iGEM2011:Free Delta Induction of Elowitz Receiver CHO]
FACS data is in for TRE:delta mcherry [TRE_Delta-mCherry dox ladder part 2|iGEM2011:TRE_Delta-mCherry dox ladder part 2] and TRE:mKate experiments [TRE_mKate dox ladder part 2|iGEM2011:TRE_mKate dox ladder part 2], however, due to loss of blue channel, cannot gate. Instead, using the top X% percent method to process data. The MATLAB code takes in the data and only keeps the top X% of data. In this case I used 50%. The program takes in filename, color to use as cutoff (in this case 'r'), and X for top X% (in this case 50).[^auto_facstop.m]
Co-Culture of Elowitz CHO cells: taken pictures also at 48 hours. Will bring to Weiss lab to process images. Redoing the experiment again, this time with 80:20 ratio as well. Will take pictures and FACS on Tuesday
CO-Culture of HEK and CHO cells:
\-Delta Heks and receiver CHO's: co-cultured Friday. added dox saturday. Will FACS Monday
\-Delta CHO's and receiver Hek's: same as above
\-TRE:delta Hek senders and CHO receivers: didn't have the resources to do just yet. Set up for experiment on Tuesday.
h5. Tyler
I just put up [iGEM2011:CHO Experiments]. I will try to do some analysis tomorrow, but some outside analysis would also be helpful.
h4. Charles
FACS of various things. CHO Experiment from Tyler had very unhealthy cells. Kens cocultures have yet to be analyzed.
h3. August 20
Divya - Miniprepped, Nanodropped, Inoculated. See Personal Notebook for details.
h3. August 19
Divya - Transformed, Inoculated. See Personal Notebook for details.
h3. August 18
Charles - FACS 100 samples in just under 90 minutes. Tyler's stuff had low concentrations so it ran slow, but data looks promising. Ken did Heks and Chos and both look promising. My stuff failed due to higher than intended initial cell concentration. Basically cells overgrew ran out of nutrients got sick and failed. Also made 20,000 ng of Dest 4-5.
Tyler \- The NCAD failed...so far. It's only been 24 hours after transfection, but I didn't see any EYFP or "clumping". I know that my transfection efficiency isn't zero because I also transfected some CHO cells on the same plate with Hef1A:EBFP2 and wew saw about 5-10% efficiency. I will check again tomorrow (48 hrs) using the microscope and then send them for FACS. Maybe it was a bad batch of DNA...
Other things I did today:
\-FACS for TRE:LacI and TRE:LacI-Krab (I'm generating graphs now)
\-Transfected CHO cells for Notch-Delta Co-culture
\-I was going to repeat Mariola's Mnt and CI434 activation experiments but I couldn't find the DNA, so I asked Divya to do some LR reactions
The NCAD failed...so far. It's only been 24 hours after transfection, but I didn't see any EYFP or "clumping". I know that my transfection efficiency isn't zero because I also transfected some CHO cells on the same plate with Hef1A:EBFP2 and wew saw about 5-10% efficiency. I will check again tomorrow (48 hrs) using the microscope and then send them for FACS. Maybe it was a bad batch of DNA...
Other things I did today:
\-FACS for TRE:LacI and TRE:LacI-Krab (I'm generating graphs now)
\-Transfected CHO cells for Notch-Delta Co-culture
\-I was going to repeat Mariola's Mnt and CI434 activation experiments but I couldn't find the DNA, so I asked Divya to do some LR reactions
\-Tyler
h3. August 17
Jon - Prepped Notch for full sequencing, minipreps, cell stocking, nanodropping, prepping trash for pickup.
Divya - Inoculated and LR'd NCADs, Nanodropped CPLRs. Will transform tomorrow. See personal notebook for details.
h3. August 16
Tiffany: Public wiki work forever. Don't use IE please.
Divya/Jon - In lab until 2am doing 26 minipreps, nanodrops, cell stocks, cleaning. :'(
Charles: Transfected cells with [EXP1-CH|iGEM2011:EXP1-CH]. Made graphs.
h3. August 15
Tyler: Prepared for transfections and updated the wiki. Will prepare CHO cells for NCAD phenotype experiment tomorrow. NCAD with HEK-293 cells failed (see [iGEM2011:NCAD Phenotype Verification]). Hef1A:GV16 experiments looked very good (see [Hef1A_GV16 Experiment|iGEM2011:Hef1A_GV16 Experiment]). TRE:LacI-EYFP-FF4 shows a lot of EYFP, about 25% increase if I remember correctly, but the LacI repression looks very weak. (see [TRE_LacI.Krab Experiments|iGEM2011:TRE_LacI.Krab Experiments]) We have a Hef1A:mKate that is bad, showing about 0-2.5% fluorescence. I will repeat these experiments tomorrow. The DRD2 shows very small levels of activation, but I think it is actually there (see [iGEM2011:GPCR Experiments]). Plan for tomorrow's transfections: 1.)TRE:LacI-Krab 2.)TRE:LacI 3.)miRFF4 4.)Lipo Ladder. Wednesday: CHO transfections.
More thoughts: The GV16 and LacI-Krab experiments were done on the same day. I got about 35% efficiency from the GV16 experiment. The LacI-EYFP-FF4 is showing about 20% when activated. I think the leakiness of rtTA3 is skewing our picture, so in the next round of LacI experiments, I will also include \+/\- Hef1A:rtTA3 as an added control.
Kenneth: My apologies, but I can't make the morning meeting. Made the line chart for TRE:eBFP experiment: [TRE_eBFP2 verification|iGEM2011:TRE_eBFP2 verification] looks good. Processed data from TRE:mKate experiment: [TRE_mKate verification|iGEM2011:TRE_mKate verification]. Looks weird. No increase in red, but increase in blue, almost like TRE:eBFP2. Not quite sure what went wrong here. Maybe I aliquoted the wrong DNA, maybe there's some mixup? Either way, has to be redone. Future directions: continue working on the matlab code, process FACS data for co-cultures and AVPR2 testing as well as free Delta (all done Sunday). Experiments planned:
1) Elowitz CHO cells [Initial CHO images - No Fluorescence] (so the cells don't express Delta-mCHerry without dox.) will FACS all of this Wednesday with proper scatter gating for CHO's
a. Dox induction of Delta (get a good curve showing conc of dox to amt of delta on surface
b. Addition of Free Delta to Notch-Gal4 cells and Notch+Delta cells
c. Co-culture with varying dox levels
d. If we see successful dox induction of Delta-mCherry and delta induction of Notch-Gal4/UAS, we can do the 2D ladder experiment where we vary both dox and delta levels for the Notch+Delta CHOs and see how much citrine, thus giving us a "surface"
2)redo TRE:Delta-mCherry dox ladder-GET GOOD DATA THIS TIME
3)TRE:eYFP and TRE:mKate need to be redone, I don't know if Charles wants to do this or not...
4)Immunostaining using no-coverslip technique for pdisplay-vasopressin as well as AVPR2 for troubleshooting
5)still waiting on free vasopressin for the AVPR2 tests.
6)test HRH4 with histamine
7)siRNA knockdown of N-cad.
Divya: Restriction Digested Clara's LRs. Send samples for sequencing. Labeled pRESC tubes. Got items from Weiss Lab.
Grant: Was amused at the bickering above. Designed quite a few primers\- this time around, for a Tango redesign and colored transactivators. Digested and gel extracted Sam's 4xFF4 gene entry backbone, pushing the Mnt-VP16-4xFF4 and Delta-mCherry-4xFF4 assemblies forward a bit. DNA work seems to have slowed considerably over the past few days\- we should make more (non-Tango) LRs. Ephrin stuff will hopefully be verified tomorrow. Takers?
Charles: Passed cells. Doing bunch of wiki work now.
Jon - Miniprepped and nanodropped 19 samples. Prepped SDMs for sequencing. Analyzed odd sequence results. Reinoculated Rescue 28 plasmids; put into incubator at 5:40pm, although many are already at high OD (wildly inconsistent though) so I'd check after a couple hours to make sure the E. coli don't reach stationary phase.
Week 12: August 22 - Later
Stay tuned!
Week 12
September 1
Grant: Inoculations of the aforementioned LR constructs.
August 29
Charles - CHLRs see CH notebook. H, HL, T, U and delta-ff4, H and T for ncad-2a-eyfp. inoculated propagations for HLNcad2Aeyfp, UASNcad2aeyfp, and Tre:delta.
August 28
Grant: Restarted LR reactions of 9 of the constructs listed on the Workflow (only the pEXPR_12_Hef1a-LacO_Notch-CI434-VP16 construct succeeded, possibly due to incubation time for LR reactions). Started PCRs for Tango constructs using new padding for TEV cut site.
August 25
Divya: Won.
(See Personal Notebook for details).
August 23
Charles - FACS Kens 50 50 and 80 20 Hek TRE-delta senders and CHO receivers. FACS was clean. Cells were all healthy. Lowered voltage of DAPI channel from 300 to 240 to move blank cells basal fluorescence to the bottom left corner. Laser was on, everything went according to plan. Preliminary results look promising, with increasing dox for each coculture, there was increasing FITC channel signal. Preliminary results suggest that our delta works.
August 22
Charles - FACS of Kens coculture stuff. Ambiguous results.







![]() ;
; "
"
