Part: BBa_I716462 – BamHI
Datasheet for Part BBa_I716462 in E. coli strain DH5alpha.
Introduction
Part I716462 was previously characterized by the UC Berkeley iGEM team in 2007. The purpose of this part was to prevent unwanted cell proliferation by engineering a genetic self-destruct mechanism into bacteria that upon induction would express a genetic material-degrading toxin, the endonuclease BamHI that would kill cells yet preserve their physical integrity. BamHI has a restriction cut site of G'GATCC, generating sticky ends (1). The restriction endonuclease was placed under the control of the arabinose- inducible promoter, pBAD. In 2007, the UC Berkeley iGEM team was able to show that cells lost their ability to reproduce but did not lyse upon induction with arabinose (2). The University of Lethbridge 2011 iGEM team wished to further characterize this part to degrade the genomic DNA of E. coli in order to have a functional chassis for our tailings pond clean-up kit without the risk of DNA contamination. In this way, tight control and regulation over our engineered bacterial cells is possible.
Characterization
Cell Survival
Materials and Methods
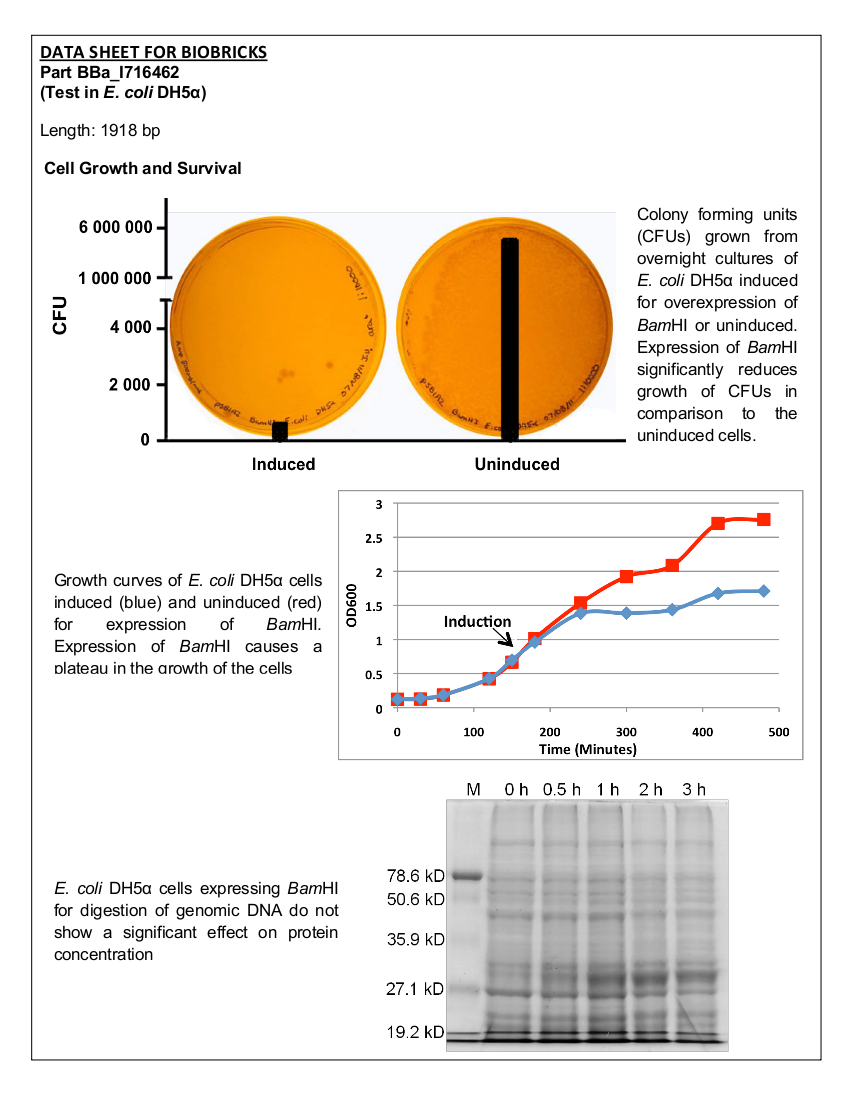
The BamHI construct was overexpressed in E. coli DH5α cells. Cultures were induced with arabinose at an OD600 of 0.6 and grown overnight. Samples of the induced culture and an uninduced culture were plated on LB agar plates to monitor growth of colony forming units.
Results
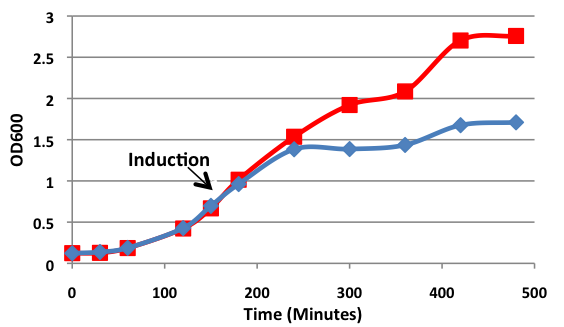
Figure 1. Growth curves of E. coli DH5α cells transformed with BamHI construct. Blue line represents cells induced at an OD600 of 0.60. Red line represents uninduced cells.

Figure 2. E.coliDH5α cells containing the BamHI construct from overnight cultures. The samples were plated on LB agar plates and the number of colony forming units (CFU) was counted. Left: culture induced for BamHI expression with arabinose, right: uninduced culture.
Analysis of Genomic DNA
Materials and Methods
E. coli DH5α cells were grown and induced to overexpress one of three samples: BamHI construct induced with arabinose (10 µM) at an OD600 of 0.6, BamHI construct uninduced, non-BamHI construct induced with arabinose (10 µM) at OD600 of 0.6. Cells were grown in LB media with 100 ng/mL ampicillin.
Qiagen Blood and Tissue Kit was used to isolate the genomic DNA of gram-negative bacteria. A 0.8% agarose gel was ran at 150 V for 90 minutes to separate the genomic DNA of E. coli DH5α cells collected at different time points (pre-induced and 30 min, 1 hr, 2 hr, and 3 hr post induction) for all three samples.
Results
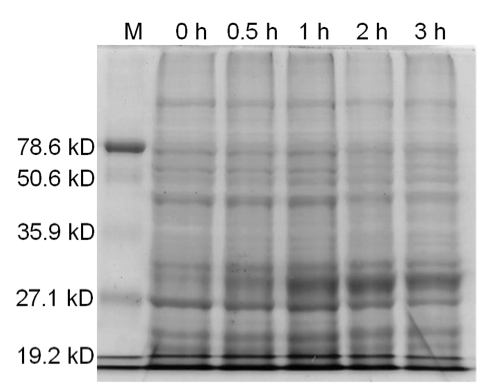
Figure 3. Isolation of genomic DNA from E. coli DH5α cells. From left to right: 1Kb + ladder, 3hr, 2 hr, 1 hr, and 30 min after induction with arabinose and pre-induction for cells containing BamHI gene construct, uninduced control, and cells not containing the BamH1 gene construct.
Conclusion
Upon induction with arabinose, E. coli DH5α cells containing the BamHI construct did not reproduce as the overexpression of the BamHI endonuclease digested the genomic DNA of the host cell. This is why in Figure 2 there are only 4 colonies seen as opposed to the control which has an uncountable amount of colonies. In Figure 1, the optical density of the BamHI expressing cells reaches a plateau; however, the same sample which has not been induced kept increasing, which indicates that the expression of BamHI is affecting normal growth of the cells.
Lastly, the genomic DNA of the E.coli cells was isolated. Upon overexpression of BamHI, there are still large segments of DNA at the pre-induced time point; however, as the post-induction time increases, there is an increase in digestion fragments.
References
(1) Viadiu H, Aggarwal AK (2000). Structure of BamHI bound to nonspecific DNA: a model for DNA sliding. Mol. Cell 5 (5): 889-895.
(2) iGEM 2007. (2011, May 10). BerkiGEM2007Present5. Retrieved from http://parts.mit.edu/igem07/index.php/BerkiGEM2007Present5.
 "
"