|
|
| (28 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| - | {{:Team:Harvard/Template:PracticeBar2}}
| + | <div id="606" style="display:none"> |
| - | {{Template:Team:Harvard/templateabouttest}}
| + | __NOTOC__ |
| - | {{Template:Team:Harvard/NotebookDataJun3}}
| + | ==June 6th== |
| | + | First day of iGEM!</div> |
| | + | <div id="607" style="display:none"> |
| | | | |
| - |
| |
| - | <html>
| |
| - | <script>
| |
| - | function show(k)
| |
| - | {
| |
| - | elem = document.getElementById('606');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==1){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - |
| |
| - | elem = document.getElementById('607');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==2){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - |
| |
| - | elem = document.getElementById('608');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==3){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - |
| |
| - | elem = document.getElementById('609');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==4){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - |
| |
| - | elem = document.getElementById('610');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==5){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - |
| |
| - | elem = document.getElementById('613');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==6){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - |
| |
| - | elem = document.getElementById('614');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==7){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - |
| |
| - | elem = document.getElementById('615');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==8){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - |
| |
| - | elem = document.getElementById('616');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==9){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - | elem = document.getElementById('617');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==10){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - | elem = document.getElementById('620');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==11){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - | elem = document.getElementById('621');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==12){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - | elem = document.getElementById('622');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==13){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - | elem = document.getElementById('623');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==14){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - | elem = document.getElementById('624');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==15){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - | elem = document.getElementById('625');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==16){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - | elem = document.getElementById('627');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==17){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - | elem = document.getElementById('628');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==18){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - | elem = document.getElementById('629');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==19){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - | elem = document.getElementById('630');
| |
| - | elem.style.display = 'none';
| |
| - | if(k==20){
| |
| - | elem.style.display = 'block';
| |
| - | }
| |
| - |
| |
| - | }
| |
| - | </script>
| |
| - |
| |
| - |
| |
| - | </style>
| |
| - | <div id="overhead">
| |
| - |
| |
| - | <div id="besedilo">
| |
| - | <div id="vse_students">
| |
| - |
| |
| - | <div id="desno_students">
| |
| - | <div id="levo_students">
| |
| - | <table style="text-align:center">
| |
| - | <caption><b>June</b></caption>
| |
| - | <tr><th>Sun</th><th>Mon</th><th>Tue</th><th>Wed</th><th>Thu</th><th>Fri</th><th>Sat</th></tr>
| |
| - | <tr><td></td><tr><td></td><td></td><td></td><td>1</a></td><td> 2</a></td><td> 3</a></td><td> 4</a></td>
| |
| - | </tr><td>5</td><td>
| |
| - | <span id="student" onclick="show(1)">
| |
| - | 6
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(2)">
| |
| - | 7
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(3)">
| |
| - | 8
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(4)">
| |
| - | 9
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(5)">
| |
| - | 10
| |
| - | </span ></td><td>11</td></tr><tr><td>12</td><td>
| |
| - | <span id="student" onclick="show(6)">
| |
| - | 13
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(7)">
| |
| - | 14
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(8)">
| |
| - | 15
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(9)">
| |
| - | 16
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(10)">
| |
| - | 17
| |
| - | </span ></td><td>18</td></tr><tr><td>19</td><td>
| |
| - | <span id="student" onclick="show(11)">
| |
| - | 20
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(12)">
| |
| - | 21
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(13)">
| |
| - | 22
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(14)">
| |
| - | 23
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(15)">
| |
| - | 24
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(16)">
| |
| - | 25
| |
| - | </span ></td></td></tr><tr><td>26</td><td>
| |
| - | <span id="student" onclick="show(17)">
| |
| - | 27
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(18)">
| |
| - | 28
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(19)">
| |
| - | 29
| |
| - | </span ></td><td>
| |
| - | <span id="student" onclick="show(20)">
| |
| - | 30
| |
| - | </span ></td></tr>
| |
| - | </table>
| |
| - | </div>
| |
| - |
| |
| - |
| |
| - | </div>
| |
| - |
| |
| - | </html>
| |
| - |
| |
| - | {{Template:Team:Harvard/NotebookDataJun2}}
| |
| - | {{Template:Team:Harvard/NotebookDataJun3}}
| |
| - |
| |
| - | <div id="606" style="display:none"></div>
| |
| - |
| |
| - | <div id="607" style="display:none">
| |
| | == June 7th == | | == June 7th == |
| | '''Miniprep of pKD42 (lambda red)''' | | '''Miniprep of pKD42 (lambda red)''' |
| Line 215: |
Line 12: |
| | Procedure: followed Qiagen Kit instructions, each student (8) using 1 mL cell suspension | | Procedure: followed Qiagen Kit instructions, each student (8) using 1 mL cell suspension |
| | | | |
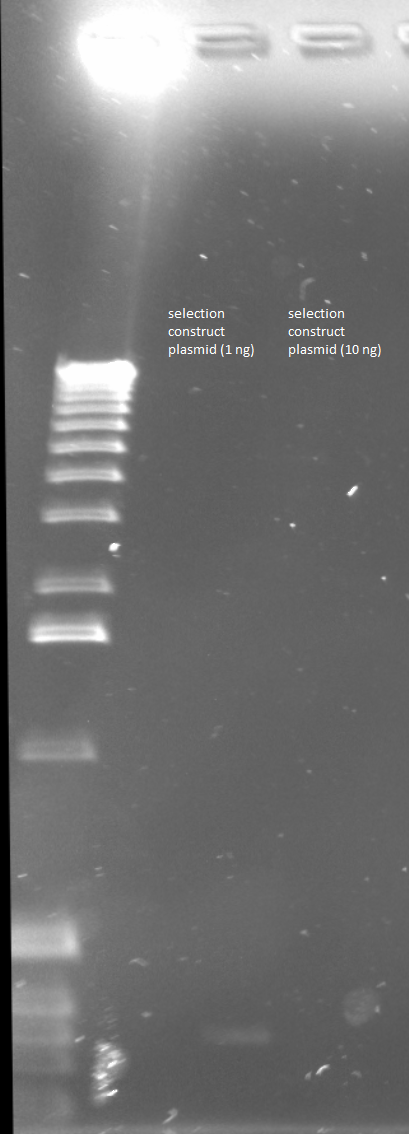
| - | Results: DNA reasonably pure (260/280 between 1.8 and 2) and between 25 and 50 ng/µL | + | Results: DNA reasonably pure (260/280 between 1.8 and 2) and between 25 and 50 ng/µL</div> |
| - | </div> | + | |
| - | | + | |
| | <div id="608" style="display:none"> | | <div id="608" style="display:none"> |
| - |
| |
| | == June 8th == | | == June 8th == |
| | '''PCR to connect ultramers into OZ052 (Zif268 F2 triplicate, GCCGATGTC)and OZ123 (Zif268 F2 triplicate, GAGTGGTTA):''' | | '''PCR to connect ultramers into OZ052 (Zif268 F2 triplicate, GCCGATGTC)and OZ123 (Zif268 F2 triplicate, GAGTGGTTA):''' |
| Line 248: |
Line 42: |
| | *5) Repeat 2-4 for 25 cycles | | *5) Repeat 2-4 for 25 cycles |
| | *6) 68⁰C for 5 min | | *6) 68⁰C for 5 min |
| - | *7) 4⁰C forever | + | *7) 4⁰C forever</div> |
| - | </div> | + | |
| - | | + | |
| | <div id="609" style="display:none"> | | <div id="609" style="display:none"> |
| - | == June 9th == | + | == June 9th - Wet Lab == |
| | *Created cell culture with selection construct (contains ZFB, His3, pyrF on plasmid) and reporter RFP (this will be used to test positive control ZFs, cells fluoresce green when ZF binds) | | *Created cell culture with selection construct (contains ZFB, His3, pyrF on plasmid) and reporter RFP (this will be used to test positive control ZFs, cells fluoresce green when ZF binds) |
| | **Picked colonies, grew in LB/amp liquid media until mid-log | | **Picked colonies, grew in LB/amp liquid media until mid-log |
| Line 265: |
Line 57: |
| | *Plated selection strain from gel stab onto tet plate. | | *Plated selection strain from gel stab onto tet plate. |
| | | | |
| - | *Began primer design for creating the kan/selection construct fusion (see our [[Primers | primer spreadsheet]] for details). | + | *Began primer design for creating the kan/selection construct fusion. |
| | | | |
| | ==June 9th - Bioinformatics== | | ==June 9th - Bioinformatics== |
| Line 418: |
Line 210: |
| | |} | | |} |
| | | | |
| - | Follow up work on this will be to convert this table to frequencies instead of values: values are less meaningful. | + | Follow up work on this will be to convert this table to frequencies instead of values: values are less meaningful. </div> |
| - | </div> | + | |
| - | | + | |
| | <div id="610" style="display:none"> | | <div id="610" style="display:none"> |
| - | == June 10th == | + | == June 10th - Wet Lab == |
| | *What we learned today: don't put E. coli plates in the -20C freezer! | | *What we learned today: don't put E. coli plates in the -20C freezer! |
| | | | |
| Line 438: |
Line 228: |
| | | | |
| | {| | | {| |
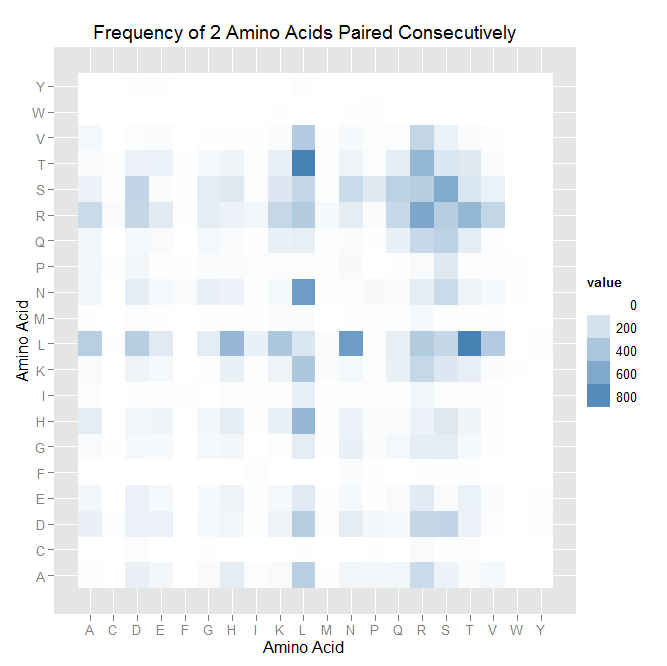
| - | | [[File:heatmap_pairing.png|thumb|left|A heatmap of the pairing data. The darker the blues indicate that the pairing occurs more often.]] | + | | [[File:HARVHeatmap_pairing.png|thumb|left|A heatmap of the pairing data. The darker the blues indicate that the pairing occurs more often.]] |
| | |} | | |} |
| | | | |
| Line 444: |
Line 234: |
| | | | |
| | {| | | {| |
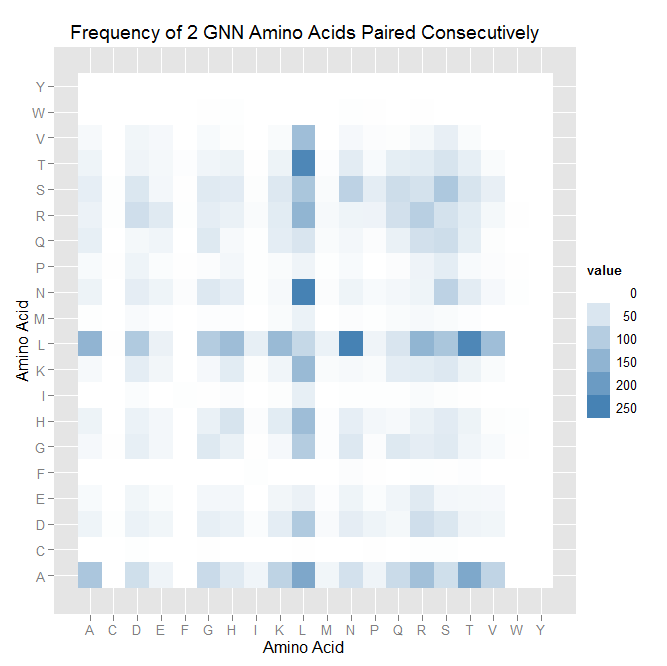
| - | | [[File:gnn_pairing_heatmap.png|thumb|left|A heatmap of the GNN pairing data.]] | + | | [[File:HARVGnn_pairing_heatmap.png|thumb|left|A heatmap of the GNN pairing data.]] |
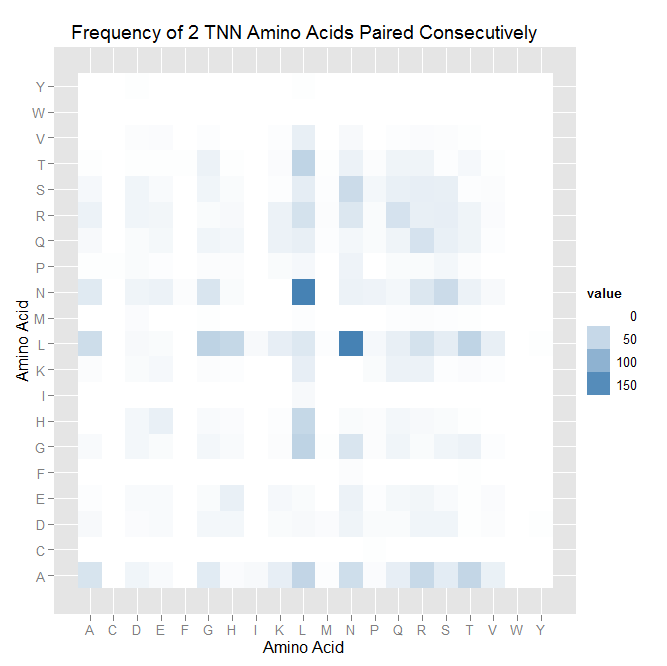
| - | | [[File:tnn_pairing_heatmap.png|thumb|left|A heatmap of the TNN pairing data.]] | + | | [[File:HARVTnn_pairing_heatmap.png|thumb|left|A heatmap of the TNN pairing data.]] |
| | |} | | |} |
| | | | |
| Line 506: |
Line 296: |
| | |} | | |} |
| | | | |
| - | Follow up work here is to check more properties, and maybe try individual pairings (ex. phobic-philic, polar-phillic). | + | Follow up work here is to check more properties, and maybe try individual pairings (ex. phobic-philic, polar-phillic).</div> |
| - | </div> | + | |
| - | | + | |
| | <div id="613" style="display:none"> | | <div id="613" style="display:none"> |
| - | == June 13th == | + | == June 13th - Wet Lab == |
| | The control zinc fingers OZ052 and OZ123 were amplified with overhanging primers to allow its insertion into the Wolfe plasmid: | | The control zinc fingers OZ052 and OZ123 were amplified with overhanging primers to allow its insertion into the Wolfe plasmid: |
| | | | |
| Line 561: |
Line 349: |
| | ===Gel images=== | | ===Gel images=== |
| | | | |
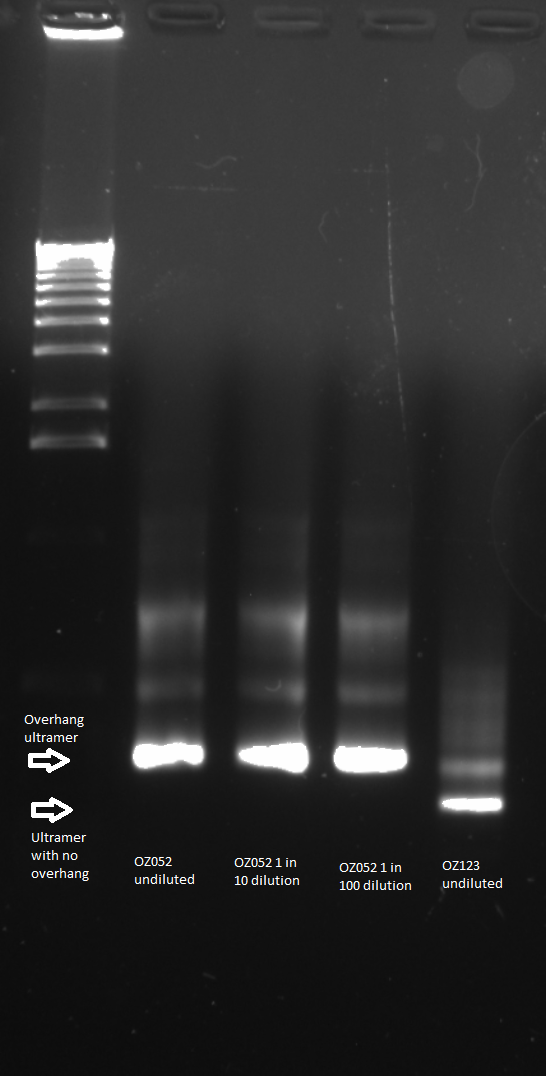
| - | [[File:2011.06.13.ultrameroverhang052,123gel1(labeled).png|thumb|left|Ultramer Overhang 6/13/11]] | + | [[File:HARV2011.06.13.ultrameroverhang052,123gel1(labeled).png|thumb|left|Ultramer Overhang 6/13/11]] |
| - | [[File:2011.06.13.wolfebackbonegel1(labeled).png|thumb|none|Backbone plasmid 6/13/11]] | + | [[File:HARV2011.06.13.wolfebackbonegel1(labeled).png|thumb|none|Backbone plasmid 6/13/11]] |
| | | | |
| | == June 13th - Bioinformatics == | | == June 13th - Bioinformatics == |
| Line 581: |
Line 369: |
| | :: add pseudo counts (call add_pseudo) and generate a dependent random call for a position (using generate_indep on the adjusted matrix) | | :: add pseudo counts (call add_pseudo) and generate a dependent random call for a position (using generate_indep on the adjusted matrix) |
| | | | |
| - | We finished generate_indep, generate_dep, and add_pseudo today, along with creating a 140x140 matrix of needed values. | + | We finished generate_indep, generate_dep, and add_pseudo today, along with creating a 140x140 matrix of needed values.</div> |
| - | </div> | + | |
| - | | + | |
| - | <div id="614" style="display:none">
| + | |
| - | == June 14 ==
| + | |
| - | *Made four LB-based media solutions, and later created glycerol stocks from these and placed in -80⁰C freezer
| + | |
| - | **Selection strain (ΔHis3ΔPyrFΔrpoZ) in 3 mL LB and 3µL of 1000x Tet solution stock
| + | |
| - | **Selection strain (ΔHis3ΔPyrFΔrpoZ) in 3 mL of LB only solution stock (control)
| + | |
| - | **Kan cassette (pZE22G) in 3 mL of LB solution stock and 3 µL of kanamycin solution stock
| + | |
| - | **Lambda Red (pKD42) in 3 mL of LB and 3 µL Amp solution stock
| + | |
| - | ***For all of these stocks, we tried to grow all to mid-log and then place them in 1,200 µL of culture and 300 µL of 80 % glycerol solution (''This is the correct protocol for creating a glycerol stock; refer to June 9th'')
| + | |
| - | ***We were only able to get the kan cassette to mid-log and created glycerol stock of the kan cassette
| + | |
| - | ***Observations included contamination of a pKD46 liquid culture, and we are leaving Lambda Red and both solutions with the selection strain for overnight growth
| + | |
| - | *Ran 1% gel (150V) with the rest of the OZ123 and OZ052 overhang PCR samples
| + | |
| - | **Used better ladder today, less the 1 kb ladder
| + | |
| - | **Bands followed the same pattern as the gel run on 6/13/11
| + | |
| - | *Used gel extraction to obtain the correct OZ123 and OZ052 PCR product from the gel
| + | |
| - | **Used Qiagen quick gel extraction kit
| + | |
| - | **OZ052 (from undiluted lane): 7.0 ng/µL, 260/280=2.42 (Note: this sample had a strange yellow substance in the column--may have been contaminated)
| + | |
| - | **OZ052 (from undiluted lane): 12.0 ng/µL, 260/280=2.02
| + | |
| - | **OZ052 (from 1:10 dilution): 10.8 ng/µL, 260/280=2.04
| + | |
| - | **OZ052 (from 1:10 dilution): 15.2 ng/µL, 260/280=1.82
| + | |
| - | **OZ052 (from 1:100 dilution): 20.6 ng/µL, 260/280=2.17
| + | |
| - | **OZ123 (from undiluted lane): 6.3 ng/µL, 260/280=2.17
| + | |
| - | *PCR the backbone fragment of the plasmid using Wolfe_R and Wolfe_L primers and a lower annealing temperature than before due to the lower melting point of Wolfe_L
| + | |
| - | **Reagents:
| + | |
| - | ***22µL Invitrogen Platinum PCR supermix
| + | |
| - | ***1µL template from a 1 in 100 dilution (1 ng)
| + | |
| - | ***1µL Wolfe_F and 1µL Wolfe_R
| + | |
| - | **94°C for 30 s
| + | |
| - | **94°C for 30 s
| + | |
| - | **53°C for 30 s
| + | |
| - | **70°C for 5 min
| + | |
| - | ***Previous three steps repeat 30 times
| + | |
| - | **70°C for 5 min
| + | |
| - | **4°C forever
| + | |
| - | *Performed a restriction enzyme digestion on the selection construct plasmid using EcoRI to test for presence/absence of inserted selection construct
| + | |
| - | **1µL EcoR1
| + | |
| - | **1µL buffer 4
| + | |
| - | **2µL backbone plasmid
| + | |
| - | **6µL ddH2O
| + | |
| - | **Incubate at 37 degrees for 90 min
| + | |
| - | **There is only one EcoR1 site (GAATTC) in the plasmid, so we should see 1 band at about 5kb
| + | |
| - | *Ran a gel (1%, 170 V) on the backbone fragment (Wolfe primers) PCR product and restriction digestion result
| + | |
| - | **Observations: the EcoR1 digest produced the expected band of around 5kb. The backbone did produce a 5kb band but also had a secondary smaller product, perhaps due to one of the primers annealing to a sequence that is a close match to its target.
| + | |
| - | *Began gradient PCR on the selection strain backbone with Wolfe_R and Wolfe_F primers because of the large difference in melting temperatures between the two
| + | |
| - | **Set the annealing temperatures within the PCR to go from 50-57 C and ran with 5 minute extension phases at 70 C
| + | |
| - | **Ran overnight
| + | |
| - | ===Today's Gel Images===
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | [[File:2011.06.14.ultrameroverhang1(labeled).png|thumb|left|Ultramer Overhang 6/14/11]]
| + | |
| - | | + | |
| - | [[File:2011.6.14.digestionbackbonePCRpaint.png|thumb|none|Digestion and PCR Backbone on Selection Construct Plasmid 6/14/11]]
| + | |
| - | | + | |
| - | == June 14 - Bioinformatics ==
| + | |
| - | | + | |
| - | We finished writing the generate function, and now have a working sequence generator. We also began more in-depth research into the 2011 work by Persikov which deals with how the zinc finger binds to DNA. He predicts several relations which we should be able to test.
| + | |
| - | | + | |
| - | | + | |
| - | Persikov sent us his SVM code (used to calculate the probability of a sequence binding to given DNA), so we also worked on adapting this to use when narrowing our sequences to those most likely to work.
| + | |
| - | *There are four canonical amino acid-base interactions involved in zinc finger interactions.
| + | |
| - | **Amino acids in positions -1, 3, and 6 on the helix are known to interact with the 3 bases of the triplet. In addition, the amino acid in position 2 interacts with the upstream base of the complementary strand (Klug 2010).
| + | |
| - | **In addition, Persikov (2011) has proposed [[#June 17 - Bioinformatics|novel interactions]] between these four amino acids based on his analysis of zinc finger binding data upto 2005.
| + | |
| - | **Persikov uses the information between these four amino acid-base interactions in his SVM, to determine whether a finger would be a good binder to a particular DNA sequence.
| + | |
| - | *In order to use his program, we need to convert the helices that are created by the generator into a format the SVM accepts.
| + | |
| - | **The SVM only considers the four canonical interactions. It assigns a numerical value to each possible amino acid-base combination. The program that converts our data into a format the SVM accepts creates a string with these numerical values based on Persikov's key. (See [[Brainstorming Notes#Input Example for SVM:|this page]] for more details on this program.)
| + | |
| - | | + | |
| - | | + | |
| - | Brandon learned basic Python today. Justin created a JavaScript program that recognizes potential binding sites from a given sequence.
| + | |
| - | </div>
| + | |
| - | | + | |
| - | <div id="615" style="display:none">
| + | |
| - | ==June 15th==
| + | |
| - | '''Gradient PCR:''' 5µL of each PCR product were run on a 1% gel. No bands appeared: the PCR appears to not have worked.
| + | |
| - | | + | |
| - | '''Selection construct:''' bacteria containing the selection construct (plasmid containing ZF, omega subunit, ZFB, His3, URA3, etc.) was made into a glycerol stock (see 6/14 and 6/9), miniprepped, and used for PCR:
| + | |
| - | *Miniprep: used Qiagen kit
| + | |
| - | **82.5ng/µL, 260/280=1.99
| + | |
| - | **91.1ng/µL, 260/280=2.01
| + | |
| - | **77.9ng/µL, 260/280=1.98
| + | |
| - | *'''PCR:'''
| + | |
| - | **PCR used to amplify section of plasmid containing zinc finger binding site, weak promoter, His3, and URA3 (with homology to join it to kan cassette)
| + | |
| - | **'''Reagents'''
| + | |
| - | **zinc finger binding site and weak promoter, selection construct plasmid:
| + | |
| - | ***1µL ZFB-wp-f (5µM) (made by 1:20 dilution of 100µM stock)
| + | |
| - | ***1µL ZFB-wp-hisura-r (5µM) (made by 1:20 dilution of 100µM stock)
| + | |
| - | ***1µL selection construct (1:100 dilution of overnight culture)
| + | |
| - | ***22.5µL of invitrogen's Platinum PCR SuperMix
| + | |
| - | **PCR used to amplify the kan cassette
| + | |
| - | **'''Reagents'''
| + | |
| - | **KAN cassette, pZE22g plasmid:
| + | |
| - | ***1µL hisura-kan-f (5µM) (made by 1:20 dilution of 100µM stock)
| + | |
| - | ***1µL kan-r (5µM) (made by 1:20 dilution of 100µM stock)
| + | |
| - | ***1µL pZE22g (1:100 dilution of glycerol stock culture)
| + | |
| - | ***22.5µL of invitrogen's Platinum PCR SuperMix
| + | |
| - | **'''Parameters:'''
| + | |
| - | *1) 94°C for 2 min (denature template, activate enzyme)
| + | |
| - | *2) 94°C for 30 sec (denature)
| + | |
| - | *3) 53°C for 30 sec (anneal)
| + | |
| - | *4) 72°C for 2 min (extend)
| + | |
| - | *5) Repeat 2-4 for 25 cycles total
| + | |
| - | *6) 72⁰C for 5 min
| + | |
| - | *7) 4°C forever
| + | |
| - | | + | |
| - | '''PCR Purification:'''
| + | |
| - | *Used the Qiagen PCR purification kit and instructions in order to purify the Kan cassette and selections construct PCR products
| + | |
| - | **Nanodrop the purification results and observed 3 ng/µL for Kan cassette and 29.8 ng/µL for ZFB-wp-His3: purification did not work well, especially for the kan
| + | |
| - | | + | |
| - | '''Selection strain (ΔHis3ΔPyrFΔrpoZ):'''
| + | |
| - | *saturated overnight culture was inoculated again: 3mL LB, 3µL tet, 30µL of overnight culture, at 37C until mid-log
| + | |
| - | *glycerol stock
| + | |
| - | *For transformation tomorrow we grew up pKD42 in 3 mL of LB, 1.5 µL of ampicillin(2000x) and one colony at '''30 C'''
| + | |
| - | **Also grew up more of the selection strain so it will be ready for electroporation transformation
| + | |
| - | | + | |
| - | '''Gel'''
| + | |
| - | *Ran gel with Kan cassette and selection construct (Binding site, His3, and URA3)
| + | |
| - | **Observations successful and image below
| + | |
| - | **Used 1 kb plus ladder
| + | |
| - | | + | |
| - | [[File:2011.06.15.kan-ZFB-wp-his3-ura3(labeled).png|thumb|Kan and ZFB-wp-his constructs 6/15/11]]
| + | |
| - | | + | |
| - | | + | |
| - | '''PCR Overlap'''
| + | |
| - | *since the purification was not very successful, we used 3µL saved from the original PCR product
| + | |
| - | *Procedure
| + | |
| - | **25µL of 2x Phusion Master Mix
| + | |
| - | **1 µL of ZFB-wp-HisURA-R (100µM)
| + | |
| - | **1µL of HisURA-Kan-F (100µM)
| + | |
| - | **21 µL of water
| + | |
| - | **1 µL Kan template and 1 µL of ZFB-wp-His3-URA3
| + | |
| - | ***4 tubes
| + | |
| - | ****Both undiluted
| + | |
| - | ****Both 1:10 dilution
| + | |
| - | ****Both 1:100 dilution
| + | |
| - | ****Both 1:1000 dilution
| + | |
| - | *Protocol
| + | |
| - | **98 C for 30 s
| + | |
| - | **98 C for 10 s
| + | |
| - | **53 C for 30 s
| + | |
| - | **72 C for 3 min
| + | |
| - | **Repeat steps 2-4 for 24 more cycles
| + | |
| - | **72 C for 5 min
| + | |
| - | **4 C 4EVA!!!
| + | |
| - | | + | |
| - | ==June 15th - Bioinformatics ==
| + | |
| - | | + | |
| - | *We continued research into Persikov's and others' work on binding.
| + | |
| - | | + | |
| - | *We worked on using the OPEN data to test Persikov's binding-predicting program: SVM
| + | |
| - | | + | |
| - | *Justin continued work on a sequence-finding program, the most up to date version can be found in the Dropbox under code/zfsitefinder.html.
| + | |
| - | *Justin and Will found 10 candidate sequences across 4 diseases that hopefully should encompass a good amount of diversity in terms of expanding the ZF library. These sequences can be found in the table below, with more details [[Media:ZF_Binding_Sequence_Candidates.xlsx|here]]. The most up to date version can be found in the Dropbox under sequences/Target Loci Sequences.
| + | |
| - | | + | |
| - | {| class="wikitable" cellpadding="5"
| + | |
| - | | align="center" style="background:#f0f0f0;"|'''Disease'''
| + | |
| - | | align="center" style="background:#f0f0f0;"|'''Target Range'''
| + | |
| - | | align="center" style="background:#f0f0f0;"|'''Binding Site Location'''
| + | |
| - | | align="center" style="background:#f0f0f0;"|'''Bottom Finger'''
| + | |
| - | | align="center" style="background:#f0f0f0;"|'''Top Finger'''
| + | |
| - | | align="center" style="background:#f0f0f0;"|'''Bottom AA (F3 to F1)'''
| + | |
| - | | align="center" style="background:#f0f0f0;"|'''Top AA (F3 to F1)'''
| + | |
| - | |-
| + | |
| - | | Colorblindness||chrX:153,403,001-153,407,000||370||GTATTTGTT||GGGCCTGCT||N/A||N/A
| + | |
| - | |-
| + | |
| - | | Colorblindness||chrX:153,403,001-153,407,000||3627||GCTGGCTGG||GCGGTAATG||EGSGLKR.EAHHLSR.#######||RRDDLTR.QRSSLVR.#######
| + | |
| - | |-
| + | |
| - | | Cystic Fibrosis||chr7:117,074,084-117,089,556||14767||GCAGGTGAT||AAAGAGCCC||QNGTLGR.EAHHLSR.#######||N/A
| + | |
| - | |-
| + | |
| - | | Familial Hypercholesterolemia||chr19:11,175,000-11,195,000||14001||GGCTGAGAC||GGAGTCCTG||ESGHLKR.QREHLTT.#######||QTTHLSR.DHSSLKR.#######
| + | |
| - | |-
| + | |
| - | | Tay-Sachs||chr15:72,674,944-72,688,031||5888||GTCTGGTCA||TCAAACTCC||DRSSLRR.RREHLTI.#######||N/A
| + | |
| - | |-
| + | |
| - | | Pancreatic Cancer||chr7:117,074,084-117,089,556||1739||GATCAAGCT||GTTTCAGTG||N/A||N/A
| + | |
| - | |}
| + | |
| - | | + | |
| - | *We collected 15 alternative zinc finger backbones (different from zif268 backbone) and their corresponding base sequences. Many of these were from Persikov 2011 and all binding sequences were confirmed on the Protein Data Bank website at http://www.pdb.org/pdb/home/home.do. The zinc finger PDB ID's and related links are:
| + | |
| - | | + | |
| - | | + | |
| - | {|border="1" cellpadding="5"
| + | |
| - | |-
| + | |
| - | ! scope="col" | PDB ID
| + | |
| - | ! scope="col" | Binding Sequence
| + | |
| - | ! scope="col" | Link
| + | |
| - | |-
| + | |
| - | |1F2I
| + | |
| - | |ATGGGCGCGCCCAT
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=1F2I]
| + | |
| - | |-
| + | |
| - | |1G2D
| + | |
| - | |GACGCTATAAAAGGAG
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=1G2D]
| + | |
| - | |-
| + | |
| - | |1G2F
| + | |
| - | |TCCTTTTATAGCGTCC
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=1G2F]
| + | |
| - | |-
| + | |
| - | |1MEY
| + | |
| - | |ATGAGGCAGAACT
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=1MEY]
| + | |
| - | |-
| + | |
| - | |1TF6
| + | |
| - | |ACGGGCCTGGTTAGTACCTGGATGGGAGACC
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=1TF6]
| + | |
| - | |-
| + | |
| - | |1UBD
| + | |
| - | |AGGGTCTCCATTTTGAAGCG
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=1UBD]
| + | |
| - | |-
| + | |
| - | |1TF6
| + | |
| - | |ACGGGCCTGGTTAGTACCTGGATGGGAGACC
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=1TF6]
| + | |
| - | |-
| + | |
| - | |1YUI
| + | |
| - | |GCCGAGAGTAC
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=1YUI]
| + | |
| - | |-
| + | |
| - | |2DRP
| + | |
| - | |CTAATAAGGATAACGTCCG
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=2DRP]
| + | |
| - | |-
| + | |
| - | |2GLI
| + | |
| - | |TTTCGTCTTGGGTGGTCCACG
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=2GLI]
| + | |
| - | |-
| + | |
| - | |2I13
| + | |
| - | |CAGATGTAGGGAAAAGCCCGGG
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=2I13]
| + | |
| - | |-
| + | |
| - | |2KMK
| + | |
| - | |CATAAATCACTGCCTA
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=2KMK]
| + | |
| - | |-
| + | |
| - | |2PRT
| + | |
| - | |CGCGGGGGCGTCTG
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=2PRT]
| + | |
| - | |-
| + | |
| - | |2WBS
| + | |
| - | |GAGGCGC
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=2WBS]
| + | |
| - | |-
| + | |
| - | |2WBU
| + | |
| - | |GAGGCGTGGC
| + | |
| - | |[http://www.pdb.org/pdb/explore/explore.do?structureId=2WBU]
| + | |
| - | |}
| + | |
| - | </div>
| + | |
| - | | + | |
| - | <div id="616" style="display:none">
| + | |
| - | ==June 16th==
| + | |
| - | | + | |
| - | *So there was totally a crazy bee hive outside today!!
| + | |
| - | | + | |
| - | '''Glycerol Stock pKD42'''
| + | |
| - | *Grew up pKD42 in 30 C and once reached mid-log created glycerol stock and placed in -80 refrigerator
| + | |
| - | | + | |
| - | '''Overlap PCR gel'''
| + | |
| - | *Ran gel to test if overlap PCR that ran through the night worked, and it did not
| + | |
| - | **Used PCR product without purification which gives good explanation for why it didn't work
| + | |
| - | | + | |
| - | *'''PCR:''' Since the PCR done the previous day (6/15) we made a back up PCR using phusion mastermix (Finnzyme)
| + | |
| - | **PCR used to amplify section of plasmid containing zinc finger binding site, weak promoter, His3, and URA3 (with homology to join it to kan cassette)
| + | |
| - | **'''Reagents'''
| + | |
| - | **zinc finger binding site and weak promoter, selection construct plasmid:
| + | |
| - | ***1µL ZFB-wp-f (100µM) (taken directly from the primer tube)
| + | |
| - | ***1µL ZFB-wp-hisura-r (100µM) (taken directly from the primer tube)
| + | |
| - | ***2µL selection construct (1:100 dilution of overnight culture)
| + | |
| - | ***25µL Phusion High-Fidelity PCR Master Mix
| + | |
| - | ***21µL distilled water (for total volume of 50µL)
| + | |
| - | **PCR used to amplify the kan cassette
| + | |
| - | **'''Reagents'''
| + | |
| - | **KAN cassette, pZE22g plasmid:
| + | |
| - | ***1µL hisura-kan-f (100µM) (taken directly from the primer tube)
| + | |
| - | ***1µL kan-r (100µM) (taken directly from the primer tube)
| + | |
| - | ***2µL pZE22g (1:100 dilution of glycerol stock culture)
| + | |
| - | ***25µL of Phusion High-Fidelity PCR Master Mix
| + | |
| - | ***21µL distilled water (for total volume of 50µL)
| + | |
| - | **'''Parameters:'''
| + | |
| - | ***1) 94°C for 2 min (denature template, activate enzyme)
| + | |
| - | ***2) 94°C for 30 sec (denature)
| + | |
| - | ***3) 53°C for 30 sec (anneal)
| + | |
| - | ***4) 72°C for 2 min (extend)
| + | |
| - | ***5) Repeat 2-4 for 25 cycles total
| + | |
| - | ***6) 72⁰C for 5 min
| + | |
| - | ***7) 4°C forever
| + | |
| - | *Ran PCR product on a gel: bands of the correct size were observed, though the kan band was much fainter than the ZFB-wp-his3
| + | |
| - | | + | |
| - | | + | |
| - | [[File:2011.06.16.kan,ZFB-wp-his-ura1|thumb|none|Kan cassette and ZFB-wp-his3 constructs 6/16/11]]
| + | |
| - | | + | |
| - | | + | |
| - | *'''Repeat PCR''' (to get a higher concentration of the Kan cassette and ZFB-wp-his3 constructs)
| + | |
| - | **same as the above backup PCR (since it was successful), but to a 4x total volume of 200µL, compared to 50µL
| + | |
| - | **PCR products were run on a gel: the correct bands were observed--see image below ("second gel")
| + | |
| - | **PCR product purification: followed Qiagen kit instructions. Strangely, the conc. was 64.6 ng/µL for Kan, purity 2.09 (260/280) and for ZFB-wp-his3, the conc. was 23.7ng/µL and the purity was 1.92 (260/280).
| + | |
| - | | + | |
| - | | + | |
| - | [[File:2011.6.16.kan&selectionconstruct(labeled).png|thumb|none|Kan cassette and ZFB-wp-his3 constructs second gel 6/16/11]]
| + | |
| - | | + | |
| - | | + | |
| - | '''Overlap PCR:''' used the kan cassette (64.6 ng/µL) and ZFB-wp-his3-ura3 (23.7 ng/µL) purified above
| + | |
| - | *1 µL of kan and 1 µL of ZFB-wp-his3-ura in each tube according to the following conditions:
| + | |
| - | **two tubes: undiluted
| + | |
| - | **two tubes: both diluted 1 in 10
| + | |
| - | **two tubes: both diluted 1 in 100
| + | |
| - | *12.5µL Phusion master mix
| + | |
| - | *8 µL ddH2O
| + | |
| - | *primers: 1.25 µL ZFB-wp-hisura_r (10 µM) and 1.25 µL hisura-kan_f (10 µM)
| + | |
| - | **we tried two different reaction types: one added the primers as usual before starting the PCR reaction, the other added the primers after 10 PCR cycles (allowing the polymerase to first use the overlapping kan and ZFB to elongate, and then the primers)
| + | |
| - | *Parameters for PCR starting with primers:
| + | |
| - | **(PCR machine 5, program name EXT3KB in IGEM folder)
| + | |
| - | **1) 98°C for 1 min (denature template, activate enzyme)
| + | |
| - | **2) 98°C for 15 sec (denature)
| + | |
| - | **3) 65°C for 15 sec (anneal)
| + | |
| - | **4) 72°C for 2 min (extend)
| + | |
| - | **5) Repeat 2-4 for 30 cycles total
| + | |
| - | **6) 72⁰C for 5 min
| + | |
| - | **7) 4°C forever
| + | |
| - | *Parameters for PCR starting without primers:
| + | |
| - | **1) 98C for 1 min
| + | |
| - | **2) 98C for 15 sec
| + | |
| - | **3) 65C for 15 sec
| + | |
| - | **4) 72C for 1 min
| + | |
| - | **5) back to step 2 for 10 cycles (PCR paused after 10 and primers added)
| + | |
| - | **6) 98C for 15 sec
| + | |
| - | **7) 65C for 15 sec
| + | |
| - | **8) 72C for 2 min
| + | |
| - | **9) back to step 6 for 20 cycles
| + | |
| - | **10) 72C for 5 min
| + | |
| - | **11) 4C forever
| + | |
| - | | + | |
| - | '''Transformation'''
| + | |
| - | *Used the selection strain (ΔHis3ΔPyrFΔrpoZ) cells at mid-log and attempted to place lambda red (pKD42) plasmid into the cell
| + | |
| - | *Procedure
| + | |
| - | **Keep on ice through out whole procedure before use of the electroporation machine
| + | |
| - | **Spin 1.5 mL of mid-log cells '''at 4 C''' for 1 minute at 18000 rcf (we created two tubes through the following steps)
| + | |
| - | **Discard supernatant and resuspend with 1 mL of '''cold''' water
| + | |
| - | **Spin again and repeat for a second water wash
| + | |
| - | ***With each wash, try to get as much supernatant out as possible(even use pipette) because don't want salts to interfere with the electrical pulse
| + | |
| - | **Resuspend pellet with 50 µL of cold water
| + | |
| - | **Add 1 ng of pKD42 to one of the tubes and 45 ng of pKD 42 to the other
| + | |
| - | **Take all of the liquid in each tube and place in two separate cuvettes for electroporation
| + | |
| - | ***Make sure the electroporation machine is on the right setting (for the cuvettes we used today it was "Ec2")
| + | |
| - | ***Wipe off all water on the side of the cuvette
| + | |
| - | **Have 1 mL of LB in hand and after pulsing, immediately put LB in cuvette
| + | |
| - | **Transfer to culture tube and place in 30 C for 2 hours
| + | |
| - | **Make 4 LB/amp plates and spread E. coli using glass beads:
| + | |
| - | ***Plate 1: 10 µL of 1 ng culture
| + | |
| - | ***Plate 2: take 700 µL of 1 ng culture, spin down and remove supernatant, resuspend in about 30 µL of LB and plate
| + | |
| - | ***Plate 3: 10 µL of 45 ng culture
| + | |
| - | ***Plate 4: take 700 µL of 45 ng culture, spin down and remove supernatant, resuspend in about 30 µL of LB and plate
| + | |
| - | **Grow overnight at 30 C
| + | |
| - | | + | |
| - | '''PCR to confirm knockouts of selection strain'''
| + | |
| - | *this PCR was to confirm that the ΔHis3ΔPyrFΔrpoZ was indeed a knockout for the His3, PyrF, and rpoZ genes
| + | |
| - | *each primer set was used for two conditions: wild-type (we used a pKD42 culture) and knockout (ΔHis3ΔPyrFΔrpoZ culture, left over from transformation)
| + | |
| - | *1 µL of either wt or ko template, diluted 1:20
| + | |
| - | *12.5 µL Phusion master mix
| + | |
| - | *1.25 µL of each 10µM primer:
| + | |
| - | **test for His3:
| + | |
| - | ***1)His3_F, His3_R
| + | |
| - | ***2)His3_F, His3_internalR
| + | |
| - | **test for PyrF:
| + | |
| - | ***3)PyrF_F, PyrF_R
| + | |
| - | ***4)PyrF_F, PyrF_internalR
| + | |
| - | **test for rpoZ:
| + | |
| - | ***5)rpoZ_F, rpoZ_R
| + | |
| - | ***6)rpoZ_F, rpoZ_internalR
| + | |
| - | **test for Zeocin (there are two primer sets because we don't know what orientation the Zeocin gene is in)
| + | |
| - | ***7)Zeocin_R, rpoZ_F
| + | |
| - | ***8)Zeocin_R, rpoZ_R
| + | |
| - | *ddH2O up to 25 µL
| + | |
| - | *Parameters:
| + | |
| - | **98 C for 5 min
| + | |
| - | **98 C for 10 sec
| + | |
| - | **65 C for 25 sec
| + | |
| - | **72 C for 45 sec
| + | |
| - | **cycle 30 times
| + | |
| - | **72 C for 5 min
| + | |
| - | **4 C forever
| + | |
| - | *Results: ran PCR products out on 1% gel (see below). There were some nonspecific bands, but the PyrF and rpoZ genes do appear to be knocked out in the selection strain. His3, however, looks like it's still present--we'll test again to confirm.
| + | |
| - | | + | |
| - | [[File:2011.06.16.selectionstraintestfordeletion(labeled)|thumb|none|Gel Confirmation of Knockouts in Selection Strain 6/16/11]]
| + | |
| - | | + | |
| - | ==June 16 - Bioinformatics==
| + | |
| - | *'''Research Targets'''
| + | |
| - | #Clinically relevant targets
| + | |
| - | #Existing ZFs that bind under-represented triplets
| + | |
| - | | + | |
| - | | + | |
| - | '''Updating our programs'''
| + | |
| - | *Many of our current programs currently look at overall data or data based on specific DNA triplets (for example: 'GAT' or 'AAA'). However, in order to more easily understand some of the patterns that occur in the datasets, we want to examine broader subsets of data. For example, do different patterns appear when looking at fingers that bind to 'GNN' triplets versus 'NGN' triplets (where 'N' represents any of the 4 bases)?
| + | |
| - | **We added the capability for our programs to accept inputs with the variable 'N' by using regular expressions.
| + | |
| - | ***We can now create lists of the zinc fingers that bind to any triplet, and create interaction matrices and frequency tables for any triplet input.
| + | |
| - | </div>
| + | |
| - | | + | |
| - | <div id="617" style="display:none">
| + | |
| - | ==June 17th==
| + | |
| - | '''Update on selection strain knockout status:''' We are trying to reach Addgene to check how His3 was knocked out---instead of deleting the gene, they may have simply introduced an early stop codon. If that's the case, our gel would have the correct bands because the primers we designed can only show whether a deletion or insertion was in that locus.
| + | |
| - | | + | |
| - | '''Transformation results''': successful!!
| + | |
| - | *The only plate with colonies was the one plated with 700 µL of cells transformed with 45 ng of pKD42
| + | |
| - | *Chose a colony to grow in 3mL LB, 1.5µL amp, 30C; make glycerol stock with mid-log cells
| + | |
| - | *Plate with colonies at 4C
| + | |
| - | | + | |
| - | '''Miniprep of pZE22G:''' (to have the plasmid containing the kan cassette on hand)
| + | |
| - | *used 2 tubes of 1.5mL overnight culture, followed Qiagen kit instructions
| + | |
| - | *38.0 ng/µL, 260/280=1.99
| + | |
| - | *27.8ng/µL, 260/280=2.02
| + | |
| - | | + | |
| - | '''Overlap PCR gel and extraction''': 1%, 150V
| + | |
| - | *Results: adding the primers in after 10 cycles was much more successful than adding them at the beginning, and all three dilutions showed the expected product band (about 2.5kb). The rest of the 1:10 dilution will be run on a gel and extracted.
| + | |
| - | *11.2ng/µL, 260/280=2.10
| + | |
| - | | + | |
| - | [[File:2011.06.16.kanZFBoverlap(labeled)|thumb|none|Successful Overlap of Kan Cassette and ZFB Gel 6/17/11]]
| + | |
| - | | + | |
| - | ==June 17 - Bioinformatics==
| + | |
| - | ===Goals===
| + | |
| - | #Make BB Database in program-readable format ✓
| + | |
| - | #Edit out BB with incomplete helices ✓
| + | |
| - | #GNN, TNN, CNN, ANN frequencies
| + | |
| - | | + | |
| - | *Targets (5-10; '''8''') '''x''' Backbones (???) '''x''' Helices (≥500)=55,000
| + | |
| - | **Backbones: similar, but not ''too'' similar to zif268; more than 1-2 aa changes, but <10
| + | |
| - | **Helices fixed based on our program-- eventually saturates and levels out
| + | |
| - | ***Graph: # of var (# of tries by the computer) vs. % space covered
| + | |
| - | | + | |
| - | [[File:Interaction Map.png|frame|right|Proposed interactions between helical zinc finger residues and base pairs of the target DNA sequence (based on Persikov 2011 <cite>Persikov2011</cite>]]
| + | |
| - | | + | |
| - | ====Options for Target DNA Sequences / ZF Helices====
| + | |
| - | #F3(known) / F2(known) / '''F1(novel)'''
| + | |
| - | #F3(known) / '''F2(SNP in b<sub>1</sub> position)''' / F1(known)
| + | |
| - | #'''F3(unknown) / F2(unknown) / F2(unknown)'''
| + | |
| - | | + | |
| - | *Excluded Rare Codons (for ''E. coli'')<cite>CodonUsage OpenWetWareCodonUsage NIHRareCodonCalculator</cite>:
| + | |
| - | **CTA
| + | |
| - | **ATA
| + | |
| - | **CCC
| + | |
| - | **CGA
| + | |
| - | **CGG
| + | |
| - | **AGA
| + | |
| - | **AGG
| + | |
| - | **GGA
| + | |
| - | **GGG
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | ----
| + | |
| - | ====References====
| + | |
| - | <biblio>
| + | |
| - | #Persikov2011 pmid=21572177
| + | |
| - | #CodonUsage http://www.sci.sdsu.edu/~smaloy/MicrobialGenetics/topics/in-vitro-genetics/codon-usage.html
| + | |
| - | #OpenWetWareCodonUsage http://openwetware.org/wiki/Escherichia_coli/Codon_usage
| + | |
| - | #NIHRareCodonCalculator http://nihserver.mbi.ucla.edu/RACC/
| + | |
| - | </biblio>
| + | |
| - | </div>
| + | |
| - | | + | |
| - | | + | |
| - | <div id="620" style="display:none">
| + | |
| - | ==June 20th==
| + | |
| - | | + | |
| - | *Grew up colony of the selection strain with pKD46 in an attempt to reach mid-log and create glycerol stock
| + | |
| - | **Unable to reach mid-log, so going to leave growing over night and use saturated culture tomorrow
| + | |
| - | *Determined primers in order to piece together the omega subunit and ZFP genes into the pZE21G plasmid (spec cassette)
| + | |
| - | *Ran PCR on His3 locus and sent to GENEWIZ to be sequenced
| + | |
| - | **used the same procedure as the earler WT/KO PCR, but with 1µL undiluted template and only His_F and His_R primers
| + | |
| - | **ran 3 reactions and sent in three primers (His_F, His_R, His_internalR)
| + | |
| - | | + | |
| - | ==June 20th - Bioinformatics==
| + | |
| - | ==='''Goals for the week'''===
| + | |
| - | *Finish designing the chip, by Wednesday hopefully
| + | |
| - | **Need chip order out, takes 4 weeks
| + | |
| - | **Need all sequences by '''''Friday'''''!!!
| + | |
| - | *FIRST PRIORITY: If we can get Persikov to work, good!
| + | |
| - | **Step one: get results he’s published, get the web app to "work" with his data, then OPEN data, and finally our data
| + | |
| - | *Brainstorming session (tomorrow?) to decide how many targets/sequences
| + | |
| - | *Determine the importance of the first/second/third nucleotide positions
| + | |
| - | **Look at NGN, NTN, NAN, NCN (''Not just GNN, etc.'')
| + | |
| - | **Pick a particular GNN, plot vs. TNN- is there a pronounced difference in position 1, or -1?
| + | |
| - | | + | |
| - | ===Today===
| + | |
| - | *Testing Persikov's Data for validation
| + | |
| - | **Persikov v. himself ✓
| + | |
| - | **Persikov v. OPEN
| + | |
| - | **Persikov v. our sequences
| + | |
| - | ===Probability data===
| + | |
| - | *The following are graphs of the probability of finding each amino acid at each position on the alpha helix.
| + | |
| - | {|
| + | |
| - | | [[File:gnn_freqs.png|thumb|left|Probability data for the 783 fingers that bind to '''GNN''' triplets. Note the high probability of leucine at position 4 and arginine at position 6.]]
| + | |
| - | | [[File:tnn_probs.png|thumb|left|Probability data for the 128 fingers that bind to '''TNN''' triplets. Note the high probability of leucine at position 4.]]
| + | |
| - | | [[File:cnn_probs.png|thumb|left|Probability data for the 16 fingers that bind to '''CNN''' triplets. There may not be enough data to consider this information statistically significant]]
| + | |
| - | | [[File:ann_probs.png|thumb|left|Probability data for the 29 fingers that bind to '''ANN''' triplets. There may not be enough data to consider this information statistically significant]]
| + | |
| - | |-
| + | |
| - | | [[File:ngn_probs.png|thumb|left|Probability data for the 298 fingers that bind to '''NGN''' triplets. The position 4 leucine motif remains. There is also a high probability (> 0.5) of a histidine at position 3 and an arginine at position 6.]]
| + | |
| - | | [[File:ntn_probs.png|thumb|left|Probability data for the 177 fingers that bind to '''NTN''' triplets. The position 4 leucine motif remains.]]
| + | |
| - | | [[File:ncn_probs.png|thumb|left|Probability data for the 244 fingers that bind to '''NCN''' triplets. The position 4 leucine motif remains. There is also a very high probability of an arginine at position 6.]]
| + | |
| - | | [[File:nan_probs.png|thumb|left|Probability data for the 248 fingers that bind to '''NAN''' triplets. The position 4 leucine motif remains. There is also a very high probability (> 0.75) of an asparagine at position 3 and an arginine at position 6.]]
| + | |
| - | |-
| + | |
| - | | [[File:nng_probs.png|thumb|left|Probability data for the 234 fingers that bind to '''NNG''' triplets. The position 4 leucine motif remains. There is also a very high probability (> 0.75) of an asparagine at position 1 and a high probability (> 0.5) of an aspartic acid at position 2 and an arginine at position 6.]]
| + | |
| - | | [[File:nnt_probs.png|thumb|left|Probability data for the 247 fingers that bind to '''NNT''' triplets. The position 4 leucine motif remains. There is also a high (> 0.5) probability of an arginine at position 6.]]
| + | |
| - | | [[File:nnc_probs.png|thumb|left|Probability data for the 262 fingers that bind to '''NNC''' triplets. The position 4 leucine motif remains. There is also a very high (> 0.75) probability of an arginine at position 6.]]
| + | |
| - | | [[File:nna_probs.png|thumb|left|Probability data for the 218 fingers that bind to '''NNA''' triplets. The position 4 leucine motif remains. There is also a very high (> 0.75) probability of a glutamine at position -1 and an arginine at position 6.]]
| + | |
| - | |}
| + | |
| - | </div>
| + | |
| - | | + | |
| - | | + | |
| - | <div id="621" style="display:none">
| + | |
| - | ==June 21st==
| + | |
| - | | + | |
| - | '''His3 sequencing results:'''
| + | |
| - | | + | |
| - | The sequencing results showed that the His3 (HisB) gene is still present in the strain and without any early stop codons. There is a 2 aa deletion in the middle of the protein, but its purpose is unknown and the gene likely is still fully functional.
| + | |
| - | *Restreak selection strain on plate from glycerol stock--tomorrow we will PCR the His3 locus and sequence again just to be sure.
| + | |
| - | *Made oligos for MAGE to insert stop codons and make a frame shift in the endogenous His3 gene, so that if necessary we can knockout His3 ourselves.
| + | |
| - | | + | |
| - | '''Selection strain with lambda red:'''
| + | |
| - | *Reinoculated and made glycerol stock
| + | |
| - | *prepared for MAGE tomorrow
| + | |
| - | | + | |
| - | ==June 21st - Bioinformatics==
| + | |
| - | ===Persikov Statistics - Graphs===
| + | |
| - | {|
| + | |
| - | | [[File:Scatterplot of top bottom 20 with SVM polynomial.png|thumb|left|Scatterplot of top/bottom 20 with SVM polynomial]]
| + | |
| - | | [[File:Sequence by sequence (lin SVM).png|thumb|left|Sequence by sequence (lin SVM)]]
| + | |
| - | | [[File:Top_Bottom_20_ZFs_(SVM_linear).png|thumb|left|Top/Bottom 20 ZFs (SVM linear)]]
| + | |
| - | | [[File:Comparison of polynomial vs linear distribution (polynomial generally higher values).png|thumb|left|Comparison of polynomial vs. linear distribution (polynomial generally higher values)]]
| + | |
| - | |}
| + | |
| - | | + | |
| - | *FQCRICMRNFS<sub>zif268 F2 Backbone</sub>/'''''Helix F1'''''/TGEKP<sub>linker</sub>
| + | |
| - | | + | |
| - | *The Persikov data shows weak predictive power for OPEN amino acid sequences. Our conclusion is that Persikov's program is not well-suited for incorporation into our helix generator. Testing Persikov's helices in his program yeilded mostly accurate results (approximately 24/25 matched known binding information). This is an important test because it proved that we are using the program correctly and that the program is in fact working properly. However, testing the OPEN sequences in Persikov's program resulted in numerous false negative values which informed our decision not to use Persikov's program to check our own hellix-generating program.
| + | |
| - | | + | |
| - | ===Phone Call with Dan===
| + | |
| - | *How conservative/risky should we be in terms of using other backbones?
| + | |
| - | **<u>'''Conservative'''</u>
| + | |
| - | ***'''Possible Pros:'''
| + | |
| - | ****More likely to get something that will work
| + | |
| - | ****Depending on how "smart" our probabilities are (from our ZF generation algorithm), we could cover a lot of novel space without straying too far from zif268
| + | |
| - | ****''Worst Case'':Something we can show for iGEM (we covered the same ground OPEN did, and found many of the same ZFs, but with a targeted approach, a "smarter" method-- not throwing random things at it; Chip is not ours, but the program is "smarter")
| + | |
| - | ***'''Possible Cons:'''
| + | |
| - | ****Might end up covering the same ground as OPEN, but doing a "worse" job than they did
| + | |
| - | ****Less likely to discover new/groundbreaking things (i.e., TNN triplets)
| + | |
| - | **<u>'''Less Conservative'''</u>
| + | |
| - | ***Have 3-6 target sequences (we're currently going for 8)
| + | |
| - | ***More backbones from non-zif268 than zif268
| + | |
| - | ***'''Pros:'''
| + | |
| - | ****We could get luck and find something no one has ever seen before (TNN, ANN). If we throw enough things at it, we're more likely to get luck.
| + | |
| - | ***'''Cons:'''
| + | |
| - | ****''Risk:'' Many of these backbones (from entire ZF world)may NOT bind DNA (i.e., may bind proteins)
| + | |
| - | ****''Risk:'' May not find anything that binds, then the whole project is a dud
| + | |
| - | *'''What is the more important variable, helices or backbones?'''
| + | |
| - | **Helices seem to be more important, backbones of secondary importance
| + | |
| - | **Backbones: ZF's unravel DNA, open the major groove-- backbone is important here, changes the bond angle, etc. (Brandon's paper-??)
| + | |
| - | *'''''Balance''''' needed between low and high risk
| + | |
| - | **If we find backbones that we know bind DNA, greatly lowers our risk
| + | |
| - | **Limited spaces on chip: zero-sum game
| + | |
| - | **With a middle of the road approach, we diminish both benefits and risk (diminishes the benefits of the high risk approach much more than it diminishes the benefits of the conservative approach; i.e., if you're playing the lottery, you're more likely to win if you buy many more tickets)
| + | |
| - | *We need to compare probabilities of randomly-generated OPEN sequences vs. probabilities of sequences randomly generated by our program
| + | |
| - | **OPEN tries to cover all space: smaller probability
| + | |
| - | **If we have a "smarter" algorithm, we can produce fewer
| + | |
| - | **However, the idea is not to repeat OPEN, but to go somewhere else, non-GNN sequences
| + | |
| - | **'''''Remember:''''' OPEN is a ''Cell'' paper; the point of the project is not to compare ourselves to them
| + | |
| - | *If we find binders for 1-2 of our sequences, that would be awesome
| + | |
| - | **Probably we'll have some that find none, some have 10, our last one might have 1,000 hits (then, we do bioinformatics to figure out why/what those hits were)
| + | |
| - | **Point: to learn and do high-level bioinformatics, and high-tech cloning techniques in the lab
| + | |
| - | **If you do find binders, you can write a paper about it!
| + | |
| - | *We have all the resources we need right now to build our chip
| + | |
| - | **We need to pick out targets
| + | |
| - | **'''Need to decide exactly what we want for:'''
| + | |
| - | ***No. of target sequences/which ones
| + | |
| - | ***No. of helices/ which ones
| + | |
| - | ***Ratio of zif268 backbones: non-zif268 backbones
| + | |
| - | **Avoid switching Leucine out of position 4, then change other positions based on our frequencies
| + | |
| - | | + | |
| - | ===Chip Design===
| + | |
| - | *No. of sequences will be more than we can put on the chip
| + | |
| - | **Helices: essentially unlimited
| + | |
| - | ***Put more-likely-to-bind helices into the risky backbones
| + | |
| - | ***Put less-likely-to-bind helices into a zif268 backbone
| + | |
| - | *Backbones
| + | |
| - | **Maybe revert to a more targeted approach: pick backbones that we know are transcription factors (TFs), that we know bind to DNA
| + | |
| - | **''OR'' research the ZFs from the phylogenetic tree
| + | |
| - | ***Pick clades to research, see if one looks better than the other
| + | |
| - | **Why did OPEN cover so many helices, without changing the backbone, but still yield predominantly GNNs?
| + | |
| - | **If we have an idea of how the backbone might affect binding, maybe we could look into some sort of low-level modeling, etc. so that we wouldn't be grasping? Could Vatsan help with this?
| + | |
| - | ***See 2000 Wolfe paper [http://www.ncbi.nlm.nih.gov/pubmed/10940247]
| + | |
| - | **Backbones ''could'' affect interactions between fingers
| + | |
| - | **Theory: energy penalty to ZF binding-- unravels DNA when binds to it
| + | |
| - | *We have 12 target sequences
| + | |
| - | **2 per 4 diseases, 4 for the 5th disease
| + | |
| - | **If we want to be more conservative, we could throw out Type III, but it could be something cool
| + | |
| - | **'''We should have mostly Type I (CoDA argument, if this is an F2)'''
| + | |
| - | **Proposed: 3 diseases, 6 sequences
| + | |
| - | ***4 Type I (F3 and F2 known, F1 novel)
| + | |
| - | ***1 Type II (GNN, ANN, GNN)
| + | |
| - | ***1 Type III (All unknown, e.g., TNN, ANN, TNN;'''''max 1''''')
| + | |
| - | Or, for 3 diseases:
| + | |
| - | # Type I's
| + | |
| - | # Type I, Type II
| + | |
| - | # Type I, Type III
| + | |
| - | | + | |
| - | *'''<u>Clinical Targets</u>
| + | |
| - | # Colorblindness ('''Type I's''')
| + | |
| - | # Familial Hypercholesterolemia (FH) (1 in 500)
| + | |
| - | # <del>Cystic Fibrosis (CF)</del>
| + | |
| - | # <del>Tay Sachs</del>
| + | |
| - | # KRAS- (oncogene/cancer)
| + | |
| - | | + | |
| - | *'''Main goal of project''': to build outside of what is already known
| + | |
| - | **If we wanted to cure a disease only, we could just use existing ZFs (i.e., find GNN binding locations)
| + | |
| - | **Also, we lend a level of specificity for insertion/deletion
| + | |
| - | **There is the possibility that there might be some area where specificity might demand ANN codons
| + | |
| - | | + | |
| - | <u>'''Current decision on chip design:'''</u>
| + | |
| - | *We will have 6 target sequences, 2 each from colorblindness, FH, and KRAS. All are "Type I" targets (only F1 is novel) with the middle finger chosen from the CODA paper (either GNN or TNN)
| + | |
| - | **N.B.: the CB and FH sequences make up full ZF nuclease cut sites. The KRAS sites, due to the small number of GNNTNN F3F2 combos available in CODA, are separate, with the flanking ZF nuclease site added afterwards in parentheses
| + | |
| - | # GGT'''G'''GT'''A'''AG (CB)
| + | |
| - | # GGA'''G'''TC'''C'''TG (FH)
| + | |
| - | # GGC'''T'''GA'''T'''GC (KRAS) (CTGAAAATT)
| + | |
| - | # GGC'''T'''GA'''C'''AC (FH)
| + | |
| - | # GGC'''T'''GG'''A'''AT (KRAS) (GACAAGAGC)
| + | |
| - | # GTC'''G'''CC'''T'''CC (CB)
| + | |
| - | | + | |
| - | *Targets 3, 4, and 6 are similar to sequences Zif268 variants successfully bind to, so the backbones will be weighted accordingly:
| + | |
| - | **Zif268_F2 backbone: 6000 helices (per target)
| + | |
| - | **10 backbones more closely related to Zif268: 300 helices each
| + | |
| - | *Targets 1, 2, and 5 will have equal distributions of backbones:
| + | |
| - | **Zif268_F2: 3000 helices
| + | |
| - | **10 backbones closely related to Zif268: 300 each
| + | |
| - | **10 backbones more distantly related to Zif268: 300 each
| + | |
| - | | + | |
| - | ===Identifying dependencies===
| + | |
| - | *We looked at the [[#Probability data|probability graphs]] to determine which amino acid positions on the finger's helix interact with which bases.
| + | |
| - | **Some interactions are fairly well estabilished, while others have been more recently proposed (See [[#June 17 - Bioinformatics|interaction map (Persikov 2011)]])
| + | |
| - | **To identify these interactions in our own data we looked at which helix positions varied most when you changed the bases. A more rigorous way to do this is to calculate the entropy change as you change the amino acids in each position.
| + | |
| - | ***'''xNN'''(Vary base 1): Amino acid 6 changes
| + | |
| - | ***'''NxN'''(Vary base 2): Amino acid 3 changes
| + | |
| - | ***'''NNx'''(Vary base 3): Amino acid -1 and 2(?) changes
| + | |
| - | **Our program looks at dependencies between amino acids when generating sequences.
| + | |
| - | ***We decided on these amino acid dependencies, using both established data and patterns we saw in the OPEN data:
| + | |
| - | ****-1 and 2
| + | |
| - | ****2 and 1
| + | |
| - | ****6 and 5
| + | |
| - | **Because there is not much data for 'CNN' and 'ANN' sequences (with 16 and 29 known fingers that bind to each triplet, respectively), we should use pseudocounts for these sequences, so that our frequency generator is not too biased toward probabilities that may not be significant.
| + | |
| - | </div>
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | <html>
| + | |
| - | | + | |
| - | | + | |
| - | <div id="vsebina_mid_right"></div>
| + | |
| - | <div id="vsebina_foot"></div>
| + | |
| - | | + | |
| - | | + | |
| - | </div>
| + | |
| - | </div>
| + | |
| - | <div align="left" class="whitebox" style="margin-top: 20px;">
| + | |
| - | <body>
| + | |
| - | </body>
| + | |
| - | | + | |
| - | </div>
| + | |
| - | | + | |
| - | </html>
| + | |
 "
"