Team:ETH Zurich/Biology/MolecularMechanism
From 2011.igem.org
(→Definition of ribosome binding sites) |
|||
| Line 18: | Line 18: | ||
==Circuit design == | ==Circuit design == | ||
| - | [[File:ETH_states_of_bandpass.png|300px|right|thumb|'''Circuit operations for SmoColi''' exposed to medium, low and zero xylene concentration resulting in a GFP band. Triangles indicating the gradient of AHL (blue) and sensor molecule ( | + | [[File:ETH_states_of_bandpass.png|300px|right|thumb|'''Circuit operations for SmoColi''' exposed to medium, low and zero xylene concentration resulting in a GFP band. Triangles indicating the gradient of AHL (blue) and sensor molecule (violet), receptively. Non active interactions and non expressed Proteins are indicated light colors.]] |
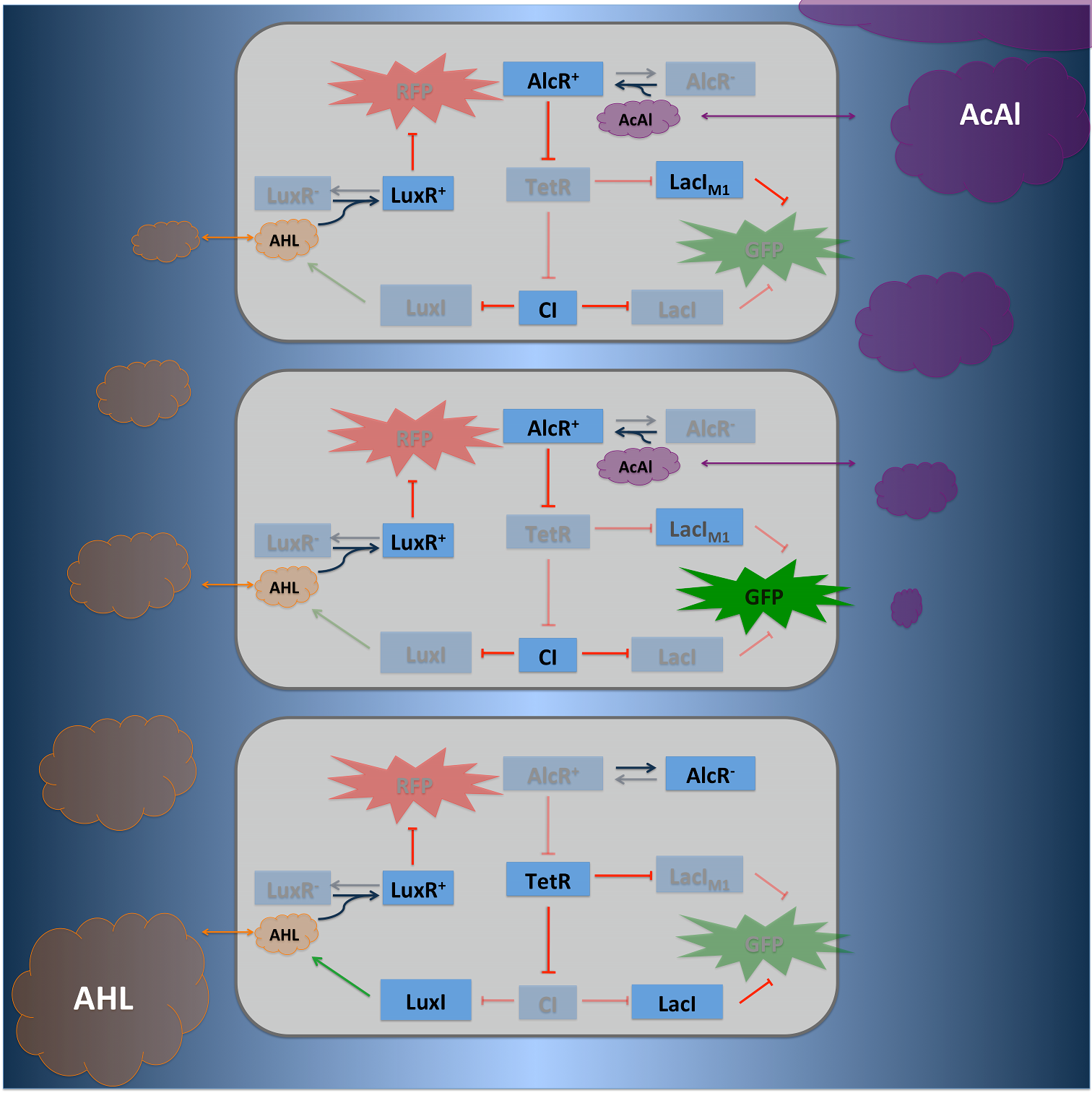
SmoColi is a bacterio quantifier which can be activated with different signal, we implemented two of them acetaldehyde and xylene. | SmoColi is a bacterio quantifier which can be activated with different signal, we implemented two of them acetaldehyde and xylene. | ||
| Line 96: | Line 96: | ||
<span id="Ref6">[6] [http://partsregistry.org/Ribosome_Binding_Sites/Prokaryotic/Constitutive/Community_Collection Registry of Standard Biological Parts '''Ribosome Binding Sites/Prokaryotic/Constitutive/Community Collection''' 21 Sep 2011, 08:49]</span> | <span id="Ref6">[6] [http://partsregistry.org/Ribosome_Binding_Sites/Prokaryotic/Constitutive/Community_Collection Registry of Standard Biological Parts '''Ribosome Binding Sites/Prokaryotic/Constitutive/Community Collection''' 21 Sep 2011, 08:49]</span> | ||
| - | + | <span id="Ref7">[7] []</span> | |
|} | |} | ||
Revision as of 08:59, 21 September 2011
| Circuit design |
| |||
| We combined a Smoke-sensitive band-pass filter with GFP output with a quorum-sensing diffusive mechanism that alarms the user of the system to high Xylene levels by expressing RFP. | ||||
Circuit designSmoColi is a bacterio quantifier which can be activated with different signal, we implemented two of them acetaldehyde and xylene. The concentration gradient which is needed for SmoColi is either naturally or achieved by synthetic cellular degradation. For example in case of Xylene we included the upper Tol pathway of Pseudomonas putida in SmoColi [1]. In contrast acetaldeydhde is naturally degraded. In our system we can use different small molceules as an activating input for the bandpass-filter [2]. The repressor which is activated by the small molecule is constantly expressed and enhances the expression of the LacIM1 repressor (codon-modified LacI) and the lambda repressor CI, which are under control of the Xyl-Promotor, respectively. High xylene concentration results in high cytoplasmic levels of CI and of LacIM1 and repression of the green fluorescent protein (GFP). Cells that are far from the input have low xylene concentrations, because of xylene degradation. Accordingly, LacIM1 and CI are only expressed at basal level. Without repression of the lamda promoter wild-type LacI is produced and represses the production of GFP. At the same point the N-Acyl homoserine lactone (AHL) Synthase LuxI is produced, AHL binds to LuxR which is constantly expressed and represses the red fluorescent protein (RFP). AHL is a quorum-sensing molecule with a high diffusion rate, it diffuses through the whole tube and represses RFP production in the whole tube, even in cells where no AHL is produced. If the xylene concentration is too high and it can not be degraded within the tube, no AHL is produced. Without AHL LuxR does not repress RFP and the whole tube turns red. To obtain better dynamics we tagged GFP and TetR with LVA tags. Finally we can also use a negative input for our system, in this case we have to introduce an additional inverter to invert the negative input signal into a positive one. Therefore the tetracycline repressor protein (TetR) was used in case of AlcR. If acetalydeyhde is present AlcR binds to the promotor of TetR and inhibit its represssion, resulting in no TetR.
|
Plasmid design Plasmid design for the AlcR system Transcriptional terminators included for each gene. Plasmid copy numbers adapted from [4]. For the biological implementation of the SmoColi system, we constructed a three-plasmid system with different antibiotic resistances. This setup was chosen in order to clone the whole SmoColi system into the same bacterial cells. The vectors used in our setup are the parts pSB6A5, pSB3C5, pSB3K3 and pSB4K5. These plasmids contain different origins of replication, thus resulting in different copy numbers of the correlating plasmid in e.coli. pBR322, p15A and pSC101 origins are used as a stable three-plasmid system [3]. The copy number influences the amount of protein produced by the cell and plays a crucial role in our design, especially for the bandpass filter. Depending on the function of a particular protein in our system we had to evaluate on which plasmid the corresponding gene should be cloned. In the xylene-responsive system we included an artificial degradation pathway (xylWCMABN) so that we could establish a xylene gradient. For this purpose the plasmid pCK04AxylR was added [1]. To avoid incompatibilities of plasmids we adapted the rest of our system and changed the composition of the genes on each vector compared to the AlcR system. |
Cloning strategyBy cloning our parts we tried to stick as much as possible to the strategies proposed in the registry of standard biological parts [5].
|
Definition of ribosome binding sitesAnother important role in the synthesis of proteins plays the ribosome binding site (RBS), where the ribosomes are bound to the mRNA at the initiation of the translation process. The ribosome binding sites which we included in our system were designed in several different ways:
The strength of all the ribosome binding sites used was estimated with a RBS calculator [7]. Although the efficiency of protein synthesis is not directly proportional to the estimated values, they can still indicate whether a certain RBS is more or less strong. |
References[1] [http://aem.asm.org/cgi/content/abstract/64/2/748 Sven Panke, Juan M. Sánchez-Romero, and Víctor de Lorenzo: Engineering of Quasi-Natural Pseudomonas putida Strains for Toluene Metabolism through an ortho-Cleavage Degradation Pathway, Appl Environ Microbiol, February 1998, 64: 748-751] [2] [http://www.nature.com/nature/journal/v434/n7037/full/nature03461.html Subhayu Basu, Yoram Gerchman1, Cynthia H. Collins, Frances H. Arnold & Ron Weiss: A synthetic multicellular system for programmed pattern formation, Nature 2005, 434: 1130-11342] [3] [http://www.springerlink.com/content/3fj1xxl9p42kx8l5/ Karl Friehs Plasmid Copy Number and Plasmid Stability, Advances in Biochemical Engineering/Biotechnology, 2004, 86: 22-192] [4] [http://openwetware.org/wiki/Arking:JCAOligoTutorial8 OpenWetWare Arking:JCAOligoTutorial8, 0 Jul 2008, 04:32 UTC. 21 Sep 2011, 01:32] [5] [http://partsregistry.org/Help:Contents Registry of Standard Biological Parts Help:contents 21 Sep 2011, 02:59] [6] [http://partsregistry.org/Ribosome_Binding_Sites/Prokaryotic/Constitutive/Community_Collection Registry of Standard Biological Parts Ribosome Binding Sites/Prokaryotic/Constitutive/Community Collection 21 Sep 2011, 08:49] [7] [] |
 "
"