Team:ETH Zurich/Biology/Detector
From 2011.igem.org
(→Where it comes from...) |
|||
| Line 13: | Line 13: | ||
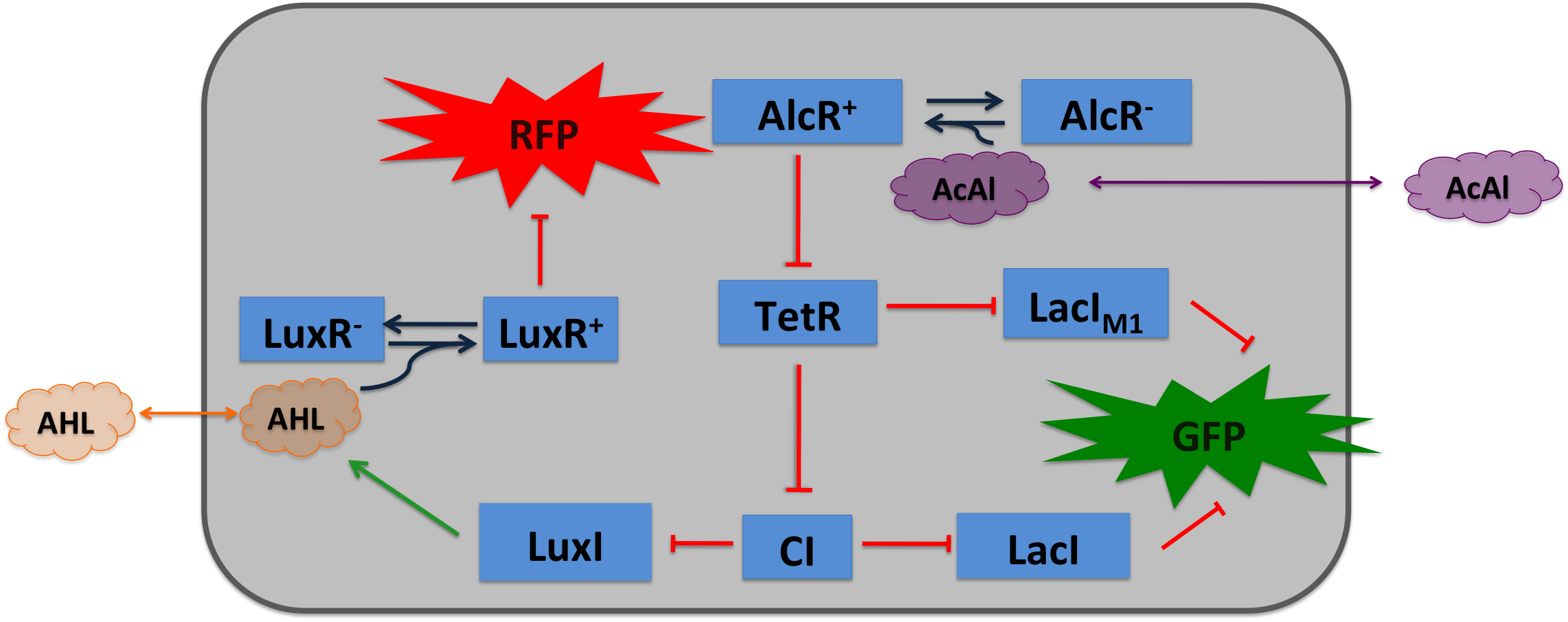
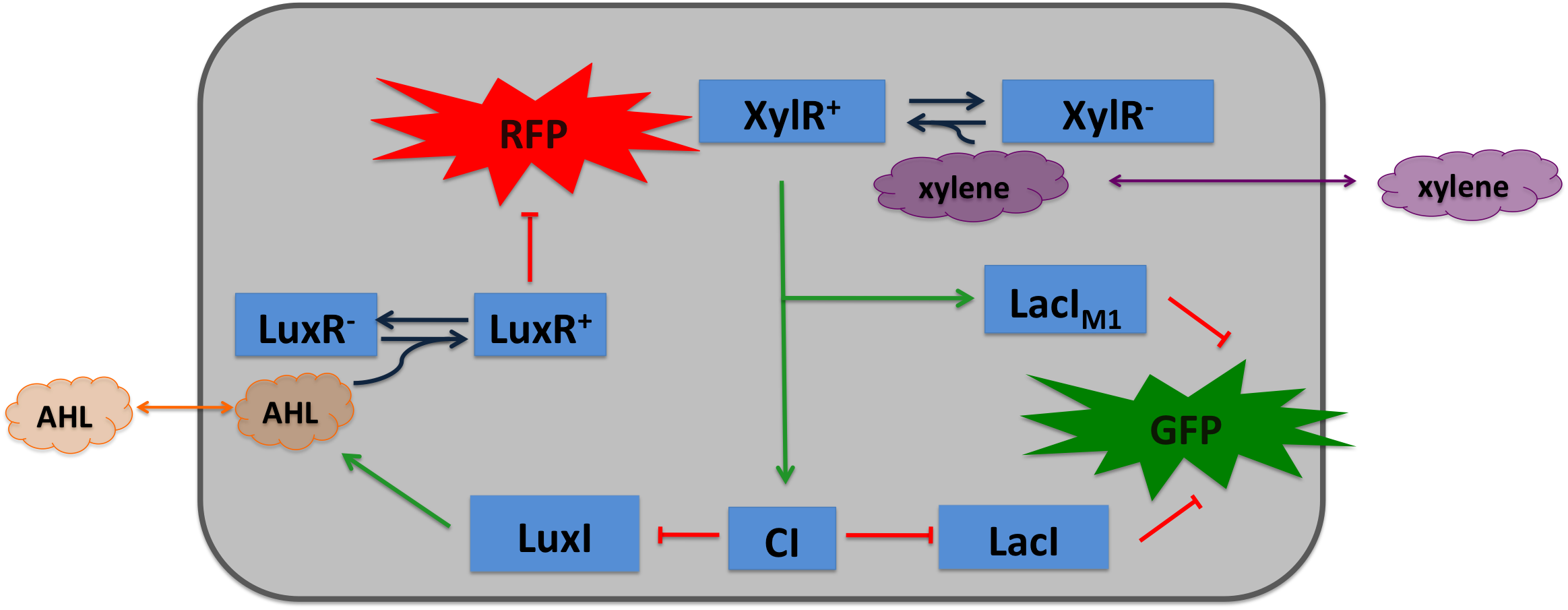
|colspan="2"|'''SmoColi consist of several different network elements: the mayor sub-networks are two sensors, a band-pass filter and a quorum sensing mechanism. | |colspan="2"|'''SmoColi consist of several different network elements: the mayor sub-networks are two sensors, a band-pass filter and a quorum sensing mechanism. | ||
| - | Cigarette smoke contains a lot of different toxic and carcinogenic components. In biology | + | Cigarette smoke contains a lot of different toxic and carcinogenic components. In biology many sensors for such components exist, mostly to induce their degradation. One is the acetaldeyhde system in ''aspergillus nidulans'': the activator AlcR binds to its operator site if acetaldeyhde is present [[#Ref1|[1]]]. Other ones are the xylene sensing system in ''Pseudomonas putida'' [[#Ref2|[2]]] and the one for styrene in ''Pseudomonas sp.'' [[#Ref3|[3]]], both also working as activators. |
We combined a smoke-sensitive band-pass filter with GFP output with a quorum-sensing diffusive mechanism that alarms the user of the system to high xylene levels by expressing RFP.''' | We combined a smoke-sensitive band-pass filter with GFP output with a quorum-sensing diffusive mechanism that alarms the user of the system to high xylene levels by expressing RFP.''' | ||
Revision as of 00:45, 22 September 2011
| Network Elements |
| ||||
| SmoColi consist of several different network elements: the mayor sub-networks are two sensors, a band-pass filter and a quorum sensing mechanism.
Cigarette smoke contains a lot of different toxic and carcinogenic components. In biology many sensors for such components exist, mostly to induce their degradation. One is the acetaldeyhde system in aspergillus nidulans: the activator AlcR binds to its operator site if acetaldeyhde is present [1]. Other ones are the xylene sensing system in Pseudomonas putida [2] and the one for styrene in Pseudomonas sp. [3], both also working as activators. We combined a smoke-sensitive band-pass filter with GFP output with a quorum-sensing diffusive mechanism that alarms the user of the system to high xylene levels by expressing RFP. | |||||
Acetaldehyde SensorWhere it comes from...In Aspergillus nidulans the alcR gene encodes a regulatory protein which induces the expression of the ethanol utilization (alc) pathway if the co-inducer acetaldehyde is present. Ethanol is converted to acetaldehyde by the alcohol dehydrogenase, acetaldehyde is afterwards metabolized to acetate by the aldehyde dehydrogenase. Both genes alcA and aldA are regulated by AlcR. AlcR also regulates its own expression by autoactivation [1].
The AlcR DNA binding domain contains a zinc bi-nuclear cluster, which can bind either to symmetric and asymmetric sites with same affinity. In contrast to other members of the Zn2Cys6, the DNA binding domain is a 16 residue long sequence between the third and fourth cysteine. AlcR binds as a monomer, but 2 molecules can bind to inverted repeats in a noncooperative manner. For high-affinity binding additional DNA sequences upstream of the zinc cluster were identified [4].
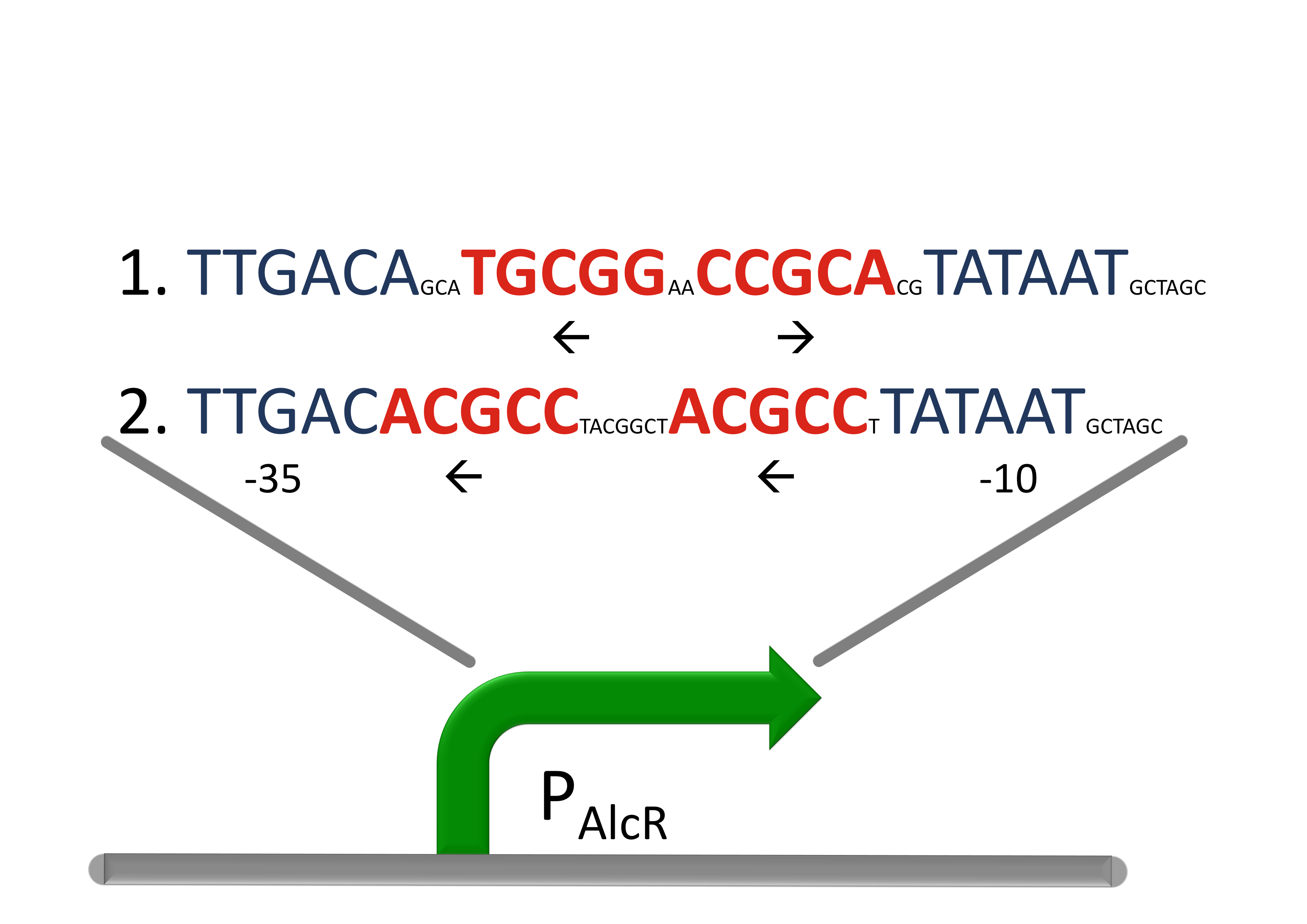
How we used itBecause fungal activators normally do not work in E. coli, we modified AlcR in a way that it acts as a repressor. The mechanism of how AlcR activates transcription is not completely known, which makes the process of redesigning it very challenging. A second challenge is the different codon usage in Apergillus nidulans and E. coli. The codon adaptation index (CAI) of Aspergillus nidulans AlcR is 0.7 in E. coli, especially at the beginning of the gene a few very rare codons were included. To get a fully E. coli-optimized gene, we ordered the gene codon-optimized. To get a partly codon-optimized version, we exchanged the rare codons at the beginning of the Aspergillus nidulans protein with PCR. For the redesigned promoter PAlcR the operator site of AlcR from Aspergillus nidulans was placed between the -10 and -35 region of the bacterial promoter. We used two different versions as DNA targets, one with direct-repeat targets and one with inverted [1]. The idea is that in presence of acetaldehyde, AlcR will bind to the operon and block the binding of the RNA polymerase. This only works if the mechanism of AlcR binding does not involve conformational changes or anything similar. To make sure that an excess of AlcR is present in respect to PAlcR, they were expressed on different plasmids with different copy numbers. The AlcR promoter is cloned in front of the TetR repressor to introduce the bandpass.
|
Xylene SensorWhere it comes from...The xylR gene in Pseudomonas putida encodes for a 566 aa long regulatory protein that activates the degradation of toluene and xylenes. In P. putida the complete upper Tol pathway is under control of the toluene-responsive promoter PU, in the presence of xylene XylR binds to PU and activates it [5]. Then the five genes xylC-xylM-xylA-xylB-xylN are expressed, where xylC encodes for the benzaldehyde dehydrogenase, xylM and xylA for subunits of the xylene oxygenase and xylB for benzyl alcohol dehydrogenase [6]. XlyN is not required for the degradation of xylene, but it is part of the whole transcription unit. The DNA binding domain of XylR binds to a 40 bp long upstream activating sequence (UAS) located 150 bp upstream from the transcription start site of the σ54 PU promoter. DNA looping between the USA and the -12/-24 motifs of the σ54-RNAP facilitates the activation of the holoenzyme by XylR [7]. How we used itXylene is not degraded naturally by E. coli. To generate a concentration gradient of xylene we engineered its degradation in SmoColi by including the upper Tol pathway. XylR is a prokaryotic enhancer binding protein which works in E. coli as well as in Pseudomonas putida. The LacIM1 and the CI regulator were under the control of the PU promoter, enhancing their expression in the presence of xylene. |
BandpassWhere it comes from...In 2005 Ron Weiss et al. constructed the first band-pass filter device in synthetic biology. Therfore he combined a high pass and a low pass filter. A input signal is proceed by cell to cell communication while the signal processing is a intracellular feed forward loop. Filters are create by using different transcriptional regulator proteins with different repression efficiency and binding affinities for corresponding promoters. How we used itWe used his pioneering network design as a basis in the planing process for our band-pass filter implementation [9]. Instead of cell-cell communication via AHL for input signal, we included our different smoke detectors which detects volatile small molecules like acetaldeyhde or xylene. |
Quorum Sensing of the AlarmsystemWhere it comes from...The luminescence operon in Vibrio fischeri is regulated by the transcriptional activator (LuxR). The lux box is a 20 bp inverted repeat sequence at a position centered 42.5 bases upstream of transcriptional start. LuxR binds to the lux box in presents of AHL and enhance its expression. LuxR is needed to activate the σ70 RNA Polymerase. The Vibrio fischeri lux operon consists of seven genes, the first one is luxI. The luxI gene encodes for the enzyme for AHL synthesis. The autoinducer AHL is secrete by the cells to coordinate gene expression based on the local density of the bacterial population. Furthermore transcription of luciferase in the lux operon is induced, leading to bioluminescence. How we used itFor our system we used a Promoter which is repressed by luxR! (BBa_R0061) It was designed by placing the lux box between and partially overlapping the consensus -35 and -10 site of an artificial lacZ promoter. In presents of AHL LuxR binds to the lux box and inhibits binding of the RNA Polymerase. [8] |
References[1] [http://www.ncbi.nlm.nih.gov/pubmed/2834622 R. Locklngton, C. Scazzocchio, D. Sequeval, M. Mathieu, B. Felenbok: Regulation of alcR, the positive regulatory gene of the ethanol utilization regulon of Aspergillus nidulans, Mol Microbiol., 1987, 1: 275-81] [2] [http://aem.asm.org/cgi/content/abstract/64/2/748 Sven Panke, Juan M. Sánchez-Romero, and Víctor de Lorenzo: Engineering of Quasi-Natural Pseudomonas putida Strains for Toluene Metabolism through an ortho-Cleavage Degradation Pathway, Appl Environ Microbiol, 1998, 64: 748-751] [3] [http://aem.asm.org/cgi/content/abstract/64/6/2032 Sven Panke, Bernard Witholt, Andreas Schmid, and Marcel G. Wubbolts: Towards a Biocatalyst for (S)-Styrene Oxide Production: Characterization of the Styrene Degradation Pathway of Pseudomonas sp. Strain VLB120, Appl Environ Microbiol, 1998, 64: 2032-2043] [4] [http://www.ncbi.nlm.nih.gov/pubmed/9182587 Lenouvel F, Nikolaev I, Felenbok B.: In vitro recognition of specific DNA targets by AlcR, a zinc binuclear cluster activator different from the other proteins of this class, J Biol Chem. 1997, 272(24):15521-6.] [5] [http://www.springerlink.com/content/r65l105rg21t1l73/ Manabu Gomada, Sachiye Inouye, Hiromasa Imaishi, Atsushi Nakazawa and Teruko Nakazawa: Analysis of an upstream regulatory sequence required for activation of the regulatory gene xylS in xylene metabolism directed by the TOL plasmid of Pseudomonas putida, Molecular and General Genetics MGG, 1992, 233:419-426] [6] [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC210316/ S Harayama, M Rekik, M Wubbolts, K Rose, R A Leppik, and K N Timmis: Characterization of five genes in the upper-pathway operon of TOL plasmid pWW0 from Pseudomonas putida and identification of the gene products, 1989, 171(9): 5048–5055] [7] [http://nar.oxfordjournals.org/content/31/23/6926.full Marc Valls and Víctor de Lorenzo: Transient XylR binding to the UAS of the Pseudomonas putida σ54 promoter Pu revealed with high intensity UV footprinting in vivo, Nucl. Acids Res., 2003, 31 (23): 6926-6934] [8] [http://jb.asm.org/cgi/content/abstract/182/3/805 Kristi A. Egland and E. P. Greenberg: Conversion of the Vibrio fischeri Transcriptional Activator, LuxR, to a Repressor , Journal of Bacteriology, 2000, 182: 805-811] [9] [http://www.nature.com/nature/journal/v434/n7037/full/nature03461.html Subhayu Basu, Yoram Gerchman1, Cynthia H. Collins, Frances H. Arnold & Ron Weiss: A synthetic multicellular system for programmed pattern formation, Nature 2005, 434: 1130-11342]
|
 "
"