Team:Cambridge/Project/prelim
From 2011.igem.org
| Line 2: | Line 2: | ||
=Preliminary observations= | =Preliminary observations= | ||
| - | In order to get a real sense of what we were looking to achieve in our project, we felt that it was important to make some observations of native squid reflectin ''in vivo''. We therefore obtained several specimens of '' | + | In order to get a real sense of what we were looking to achieve in our project, we felt that it was important to make some observations of native squid reflectin ''in vivo''. We therefore obtained several specimens of ''Loligo opalescens'' and ''Loligo vulgaris'' squid from a local seafood restaurant and an online fishing bait store for dissection. We chose these species because the whole family of loliginid squid has been identified to contain reflectin, and these particular species were the only members of the family available to us. |
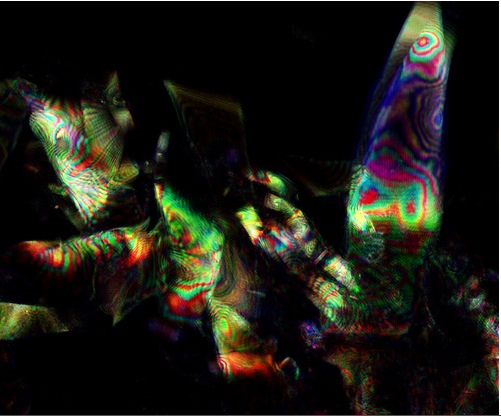
We used a confocal microscope to observe iridescent behaviour in eye and mantle tissue, by the following [https://2011.igem.org/Team:Cambridge/Protocols/Confocal_Microscopy_of_Loligo_Eye_and_Mantle_Dermis_Samples protocol]. The stunning images produced provided a very useful reference to help us to identify what recombinant (well folded) reflectin could look like in E. coli, and definitely enthused the team to obtain bactiridescence! | We used a confocal microscope to observe iridescent behaviour in eye and mantle tissue, by the following [https://2011.igem.org/Team:Cambridge/Protocols/Confocal_Microscopy_of_Loligo_Eye_and_Mantle_Dermis_Samples protocol]. The stunning images produced provided a very useful reference to help us to identify what recombinant (well folded) reflectin could look like in E. coli, and definitely enthused the team to obtain bactiridescence! | ||
| + | |||
| + | ===Theory=== | ||
| + | [[File:Squideye reflectin250repeat.gif| 300px | thumb | right| This image is a gif composed of layers taken as the depth of the focal plane was altered (via moving the stage of the microscope) through a sample of reflective squid eye cells mounted in Phosphate buffered saline]] | ||
| + | Confocal microscopy uses a pinhole aperture to direct light to a small region of a sample. Illuminating one small point at a time reduces the scattering of light which would normally obscure an image. Light is collected via another pinhole. This second pinhole further enhances the image because it only permits the entry of light that is directly from the focal point. Due to the punctate nature of the imaging process, images must be reconstructed, in modern confocal microscopes a computer assembles the array of pixels into an on screen image. As described above we set the microscope to collect light reflected from the sample as we were searching for iridescence in the samples.Paul Grant optimised the settings and the images were overlaid. | ||
| + | |||
| + | We are very grateful to Fernan Federici who helped us, taking this image using the 405nm, 488nm, 633nm laser beams and with the pinhole opened to a wider aperture. | ||
| + | [[File:Iridescent cells from around squid eye.jpg |thumb| 300px| left| Iridescent cells from around the squid eye, with thanks to Fernan Federici and Paul Grant]] | ||
| + | |||
{{Template:Team:Cambridge/CAM_2011_TEMPLATE_FOOT}} | {{Template:Team:Cambridge/CAM_2011_TEMPLATE_FOOT}} | ||
Revision as of 15:51, 10 August 2011
Preliminary observations
In order to get a real sense of what we were looking to achieve in our project, we felt that it was important to make some observations of native squid reflectin in vivo. We therefore obtained several specimens of Loligo opalescens and Loligo vulgaris squid from a local seafood restaurant and an online fishing bait store for dissection. We chose these species because the whole family of loliginid squid has been identified to contain reflectin, and these particular species were the only members of the family available to us. We used a confocal microscope to observe iridescent behaviour in eye and mantle tissue, by the following protocol. The stunning images produced provided a very useful reference to help us to identify what recombinant (well folded) reflectin could look like in E. coli, and definitely enthused the team to obtain bactiridescence!
Theory
Confocal microscopy uses a pinhole aperture to direct light to a small region of a sample. Illuminating one small point at a time reduces the scattering of light which would normally obscure an image. Light is collected via another pinhole. This second pinhole further enhances the image because it only permits the entry of light that is directly from the focal point. Due to the punctate nature of the imaging process, images must be reconstructed, in modern confocal microscopes a computer assembles the array of pixels into an on screen image. As described above we set the microscope to collect light reflected from the sample as we were searching for iridescence in the samples.Paul Grant optimised the settings and the images were overlaid.
We are very grateful to Fernan Federici who helped us, taking this image using the 405nm, 488nm, 633nm laser beams and with the pinhole opened to a wider aperture.
 "
"