Team:Cambridge/Experiments/Protein Purification
From 2011.igem.org
(→Practice) |
(→Results) |
||
| Line 11: | Line 11: | ||
==Results== | ==Results== | ||
| - | Photospectroscopy readings from the eluted solution indicated that we had obtained approximately 0.4mg of reflectin from the column for the denser culture, and 0.2mg from the other. After dialysis, a vacuum centrifuge was used to precipitate the protein. this precipitate was taken to the Nanophotonics centre in order to make our first reflectin thin films, and SDS-PAGE was run by another member of the department to check whether or not reflectin was contained in the precipitate. | + | Photospectroscopy readings from the eluted solution indicated that we had obtained approximately 0.4mg of reflectin from the column for the denser culture, and 0.2mg from the other. After dialysis, a vacuum centrifuge was used to precipitate the protein. this precipitate was taken to the Nanophotonics centre in order to make our first reflectin thin films, and SDS-PAGE was run by another member of the department to check whether or not reflectin was contained in the precipitate. |
| + | |||
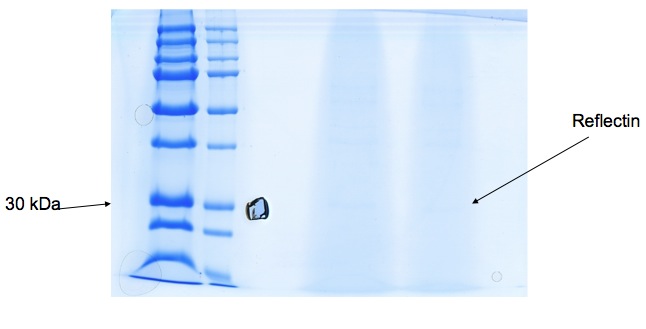
| + | We received gel image (see below) back from Kathryn Lilley who kindly offered to run a protein gel for us. It's not initally clear, but a band at 30-36kDa suggests Reflectin may be present. We're hoping to use mass spectrometry to confirm this tentative result. | ||
| + | |||
| + | [[File:Cam Reflectin Gel1.jpg | thumb | 600px | center ]] | ||
| + | |||
| + | Despite not being certain we had reflectin, we pressed on with our [thin films experiment], which produced some very interesting results! | ||
Since this was the first time any of us had tried to perform the his-trap technique, we also learnt some valuable lessons from our experiences in this first experiment. Some helpful 'tips' have been added to the [https://2011.igem.org/Team:Cambridge/Protocols/Protein_Purification his-trap purification protocol] as a result, which really helped us to improve our yield of reflectin using this method. | Since this was the first time any of us had tried to perform the his-trap technique, we also learnt some valuable lessons from our experiences in this first experiment. Some helpful 'tips' have been added to the [https://2011.igem.org/Team:Cambridge/Protocols/Protein_Purification his-trap purification protocol] as a result, which really helped us to improve our yield of reflectin using this method. | ||
{{Template:Team:Cambridge/CAM_2011_TEMPLATE_FOOT}} | {{Template:Team:Cambridge/CAM_2011_TEMPLATE_FOOT}} | ||
Revision as of 10:39, 9 September 2011
His-Trap Protein Purification - First Attempt
Bacteria expressing his-tagged reflectin were lysed, and the protein was purified using a his-trap column and a denaturing protocol in order to solubilise reflectin.
Practice
- After high copy expression plasmids for his-tagged reflectin were successfully assembled and transformed in E. coli, cell cultures were incubated overnight with 1mM arabinose to induce reflectin expression.
- Buffers were prepared, and their pH checked and readjusted if necessary on the day of purification.
- An inclusion body prep was performed with 50ml of overnight culture. Reflectin was purified from the resulting lysate using a his-trap column.
- This procedure was performed using two different cultures of the same bacteria. The culture that produced the highest yield of reflectin was selected as a 'seed' for future cultures that would be used to harvest more protein.
Results
Photospectroscopy readings from the eluted solution indicated that we had obtained approximately 0.4mg of reflectin from the column for the denser culture, and 0.2mg from the other. After dialysis, a vacuum centrifuge was used to precipitate the protein. this precipitate was taken to the Nanophotonics centre in order to make our first reflectin thin films, and SDS-PAGE was run by another member of the department to check whether or not reflectin was contained in the precipitate.
We received gel image (see below) back from Kathryn Lilley who kindly offered to run a protein gel for us. It's not initally clear, but a band at 30-36kDa suggests Reflectin may be present. We're hoping to use mass spectrometry to confirm this tentative result.
Despite not being certain we had reflectin, we pressed on with our [thin films experiment], which produced some very interesting results!
Since this was the first time any of us had tried to perform the his-trap technique, we also learnt some valuable lessons from our experiences in this first experiment. Some helpful 'tips' have been added to the his-trap purification protocol as a result, which really helped us to improve our yield of reflectin using this method.
 "
"