Team:BU Wellesley Software/Notebook/EvelynNotebook
From 2011.igem.org
Contents |
Week 6/1/11-6/3/11
Participated in iGEM bootcamp. For the week we we learned bio-basics, CAD basics, Tuberculosis overview, digital logic design and clotho. For clotho we built an application that would show hello world and then we modified the app so that another user could input a part sequence that would be stored in the clotho database. Finally we had a group outing at Jillian's.
Week 6/6/11-6/10/11
On the last day of bootcamp we learned more information on synthetic biology and gave the Wellesley team a tour of the wetlab and our liquid handling robot. The next day I worked with Kyle Jones to look for constitutive promoters in the MIT Registry of Parts. We decided to use BBa_J23100, BBa_J23101, BBa_J23109, and BBa_I14053. The first three are part of the Anderson Collection and are Amp Resistant. These promoters had RBS inside their plasmids. The last constitutive promoter is Kan resistant. Vanessa Yanez and Alberto looked for induced promoters and Shannon and Margueax looked for RBSs. After locating our parts we transformed them and let them sit over night. Unfortunately the parts that we transformed did not work after the plasmid prep. We had low values for the concentration and realized that the cultures that did grow in the LB and amp solution was not E coli. In order to confirm our suspicions we looked at the culture under a light microscope. My cultures seemed to have string like cells which we concluded to be contamination. Only 1 culture worked out of the 18 that were performed that week. Finally we met together to figure out how we wanted to split up the work and what exactly we wanted to do as a team. Margeaux and Alberto did mini prepreps, restriction digest, gel extraction, ligations, and finally transformations for GFP and terminators. The rest of us redid all the old procedures in search for the reason for the contamination.
Week 6/12/11-6/17/11
In order to decrease contamination we autoclaved the pipet tips and used aseptic techniques where applicable. We redid the plasmid preps for the same parts. This time we grew the Ecoli in just LB solution and then in LB with Amp. The next morning we discovered that the cells in the LB and Amp solutions did not grow while the cells in the LB solution did. This proved our suspicion that the plates were our problem. On Monday we prepped the UV inducible promoter for the QiaCube. The UV promoter was the only transformation and plasmid prep that worked. We did the transformation on the existing Amp plates but this time we used a negative control. After incubating the transformed cells we discovered that the negative control did in fact grow colonies. This lead us to making new Amp plates and redoing transformations and plasmid preps for the chosen parts. For our transformations we had two of our Anderson Constitutive promoters grow red colonies. When we did plasmid preps however they did grow in the LB and AMP solution. These parts had concentrations values ranging from 9.6 to 56.6 ng/microliter. In the meanwhile, Alberto and Margeaux cut and ligated more GFP and terminator B0015.
Timeline
Week 6/19-6/24 Create the various constructs using the parts we have finally made plasmid preps of using GFP. Once we can prove that the construct in fact worked we will test them using the RFP, EYFP, and BFP. This way we will know how to order the various fluorescent proteins using various promoters and RBS.
Week 6/27-7/1 Figure out how to use invertases and figure out how to incorporate them into our various constructs. Trouble shoot any problems we run in to and fix them. Then we can try to flip the gene with the invertase and see if it actually works.
Week 7/4-7/15 Start to organize all the parts in the order in which the comp team has chosen to build them. It will be our task to figure out how the part will work together and what we will need in order to allow the process we want it to do to happen.
Week 7/15-8/1 Finish up the desired construct and possibly explore other forms using the TB gene.
Week 6/20/11-6/24/11
Performed a restriction digest on:
1. EYFP 2. BFP 3. ECFP 4. RBS: BBa_J61124 5. RBS: BBa_J61100 6. GFP and terminator 7. YFPc 8. GFPc 9. promotor: BBa_R2000.
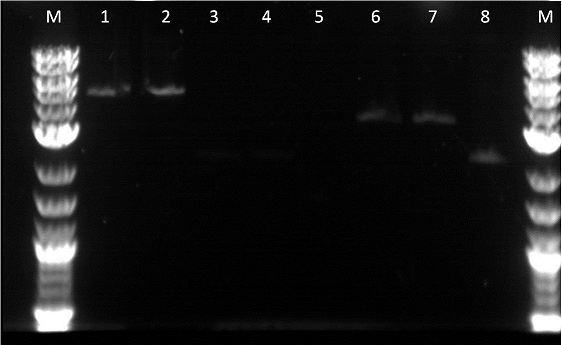
Unfortunately, as I had not used the Qiacube I ended up throwing out the gel extractions of these parts. Therefore, the parts had to be redone. However, from the gel we observed that the EYFP and ECFP were in fact cut in the restriction digest. Unfortunately, it did not seem like our GFP plus terminator worked. We could have better adjusted if we had negative controls with the uncut parts. The next morning we transformed:
1. Ptet which is BBa_R0040 in an AMP plate 2. GFP BBa_J52028 on a KAN plate
A restriction digest was also preformed on RFP, Pbad, Pcat, Terminator B0015, E0240, and E0430. A plasmid prep was then done on RBS: J61101, constitutive promoter: R2000, YFPc, and GFPc. These were incubated for about 12 hours. The next morning we made more plasmid preps on BFP, RFP, EYFP, ECFP, and finally pTEt. I preformed an mini prep using the Qiacube on the last 5 parts. I was required to centrifuge the 2ml of overnight cultures for 3 mintues for 8,000rpm then remove the supernatent. We ended having good values for the mini preps. Finally, friday the liquid handling robot was suppose to perform a restriction digest on GFP J52028 but was not able to as it could not pipet fractions. This brought the project back but allowed for more bugs to be fixed.
Week 6/27/11-7/1/11
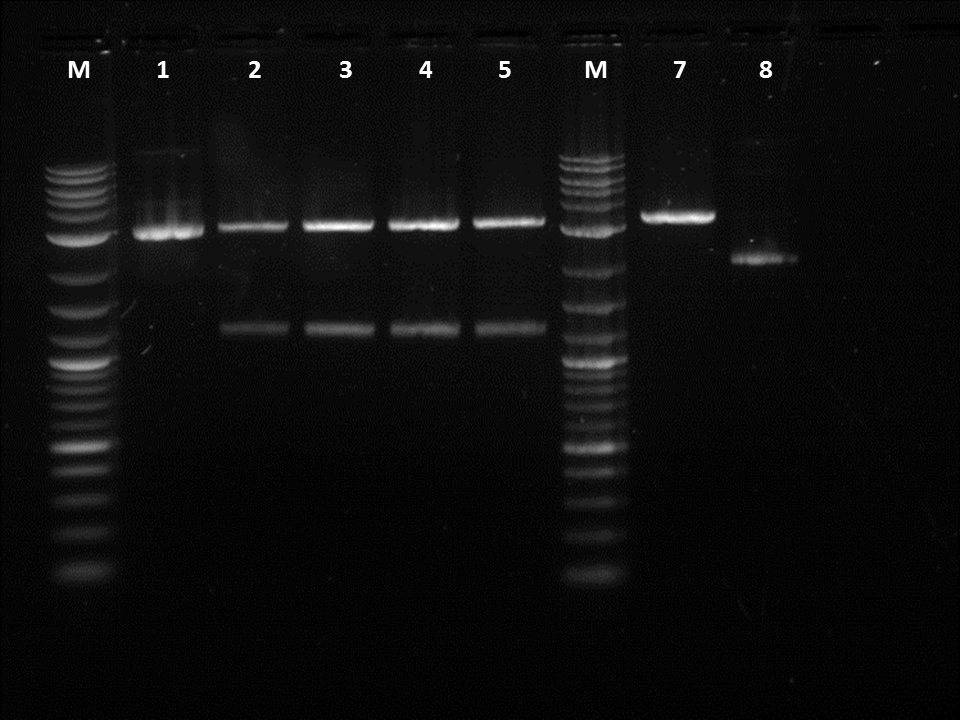
1. Cut constitutive promoter: Pcat: BBa_I14053 2. Cut constitutive promoter: Pcat: BBa_I14053 3. Cut constitutive promoter: Ptet: BBa_R0040.1 4. Cut constitutive promoter: Ptet: BBa_R0040.1 5. Uncut constitutive promoter: Ptet: BBa_R0040.1 6. Cut Terminator: BBa_B0015 7. Cut Terminator: BBa_B0015 8. Uncut Terminator: BBa_B0015
I did a restriction digest on Pcat, Ptet, and another terminator. For this procedure we doubled the amount of enzymes in order to better our results. During the restriction digest I had to incubate the cells 20 extra minutes in the 37 degree and then it was placed in the pcr machine. The bands were very unclear and we were not certain if the plasmids were cut as we did not have enough control. This mistake allowed us to realize that a control is essential as the cut promoters did not differ very much from the uncut plasmid in the number of base pairs. Also, our ladder was very bright compared to the bands and we were informed by Traci that it was the result of it being too concentrated. After cutting the bands out I had to do 3 of the gel extraction by hand. There was not enough space in the qiacube so we did one of each part in the cube and the doubles on the bench.
1. Uncut GFP: BBa_J52028 2. Cut GFP manual: BBa_J52028.2 3. Cut GFP Robot 1: BBa_J52028.2 4. Cut GFP Robot 2: BBa_J52028.2 5. Cut GFP Robot 3: BBa_J52028.2 6. Cut Terminator: BBa_B0015 7. Uncut Terminator: BBa_B0015
After fixing the issues we had with our procedures we decided to move on and do a restriction digest using our liquid handling robot. It was important to use the same liquid cultures in order to eliminate any variables other then performing the RD by hand or by the robot. Three of the restriction digests were done on the robot in order to make sure the same process could be repeated. I also did a restriction digest on a terminator so the robot could later ligate the GFP with the terminator. In order to cut the GFP we used EcoRI and SpeI while for the terminator I used EcoRI and Xbal. After running the gel, it could be seen that the GFP was cut as it was about 1.2KB on the gel. However, while loading the gel I realized that some of the RD done by the robot had a total volumes less than 50 micro-liters.
Wet Lab Protocols
These are the downloadable protocols used by the BU Wet Lab team, written in Excel 2003 format (*.xls).
 "
"